Review Article - (2022) Volume 0, Issue 0
Relationship between Acral Lentiginous Melanoma and Exposure to UV Radiation
Laura Camila Garcia Medina1*,
Maria Patricia Gamero Tafur2,
Maria Camila Marin Murillo3,
Yinneth Yasmin Ruiz Atencio4,
Rosa Angelica Osorio Burgos5,
Diana Lizeth Grajales Trujillo6,
Catalina Maria Gutierrez Pico7 and
Jonathan Oscar Reyes Padron8
1General Physician, Universidad de los Andes, Bogota, Colombia
2General Physician, Universidad del Sinu, Monteria, Colombia
3General Physician, Universidad El Bosque, Bogota, Colombia
4General Physician, Universidad del Sinu, Cartagena, Colombia
5General Physician, Universidad del Sinu, Monteria., Colombia
6General Physician, Fundacion Universitaria Juan N. Corpas, Bogota, Colombia
7General Physician, Universidad Militar Nueva Granada, Bogota, Colombia
8General Physician, Universidad del Zulia, Venezuela, Colombia
*Correspondence:
Laura Camila Garcia Medina, General Physician, Universidad de los Andes, Bogota,
Colombia,
Email:
Received: 22-Apr-2022, Manuscript No. Iphsj-22-12746;
Editor assigned: 24-Apr-2022, Pre QC No. Iphsj-22-12746 (PQ);
Reviewed: 27-May-2022, QC No. QC No. Iphsj-22-12746;
Revised: 01-Jun-2022, Manuscript No. Iphsj-22-12746(R);
Published:
09-Jun-2022, DOI: 10.36648/1791- 809X.16.S7.946
Abstract
Background: Acral melanoma is a melanoma that occurs on the hands and feet, represents 2 to 3% of all new melanomas, for its diagnosis a narrow margin excisional biopsy is recommended. Ultraviolet radiation is classified as a complete carcinogen, because it is both a mutagen and a non-specific damaging agent and has both tumor initiator and tumor promoter properties.
Methodology: A narrative review was carried out through various databases from January 2009 to January 2022; the search and selection of articles was carried out in journals indexed in English. Keywords were used: Melanoma, Acral, and Ultraviolet.
Results: Acral Lentiginous Melanoma, like mucosal melanomas, is a subtype of melanoma with low mutational load and does not present mutations induced by ultraviolet radiation that are commonly observed in other cutaneous malignant melanomas. Stress or shear force is considered one of the factors for the induction of Acral Lentiginous Melanoma, since the incidence is higher in the weight-bearing areas of the foot.
Conclusions: This review offers updated and detailed information on the association between acral litigious melanoma and ultraviolet exposure, as well as other factors as its triggers.
Keywords
Melanoma; Acral; Ultraviolet
Introduction
Acral lentiginous melanoma, also sometimes simply called acral
melanoma, is melanoma that occurs on the hands and feet
(palms, soles, fingers, toes, and nail units). The word acral derives
from the Greek word referring to the uppermost or uppermost
portion of the limbs [1-5].
The term melanoma refers to a malignancy of melanocytes, the
pigment-producing cells in the epidermis. Acral melanoma was
first separated as a distinct subtype of cutaneous malignant
melanoma in 1977.
Acral lentiginous melanoma accounts for 2 to 3% of all new
melanomas. The average age of diagnosis of this disease is
62.8 years. The incidence of acral melanoma in all populations
increases with age, with a sharp increase in incidence per person
per year after age 80 years. It affects a similar number of men and
women; however women are more commonly diagnosed at an
earlier stage than men. Acral melanoma has a disproportionately
higher incidence in non-white patients compared to other
melanoma subtypes [6-8].
For clinically suspected melanomas, a narrow-margin excisional
biopsy is recommended; however, it is recognized in the
recommendations that this may not be practical in an acral site.
Thus, a partial sample of the lesion is considered an acceptable
alternative, although the main risk with the partial sample is that
it may result in an inaccurate histological diagnosis. A sample
should be taken from the most nodular portion of the lesion in
order to determine an accurate Breslow depth [9-10].
Ultraviolet radiation is classified as a "complete carcinogen"
because it is both a mutagen and a nonspecific damaging agent
and has both tumor-initiating and tumor-promoting properties
[12]. In environmental abundance, ultraviolet rays are the most
important modifiable risk factor for skin cancer and many other
environmentally influenced skin disorders [11, 12].
However, UV rays also benefit human health by mediating the
natural synthesis of vitamin D and endorphins in the skin, thus
UV rays have complex and mixed effects on human health [9, 12].
However, excessive exposure to ultraviolet rays carries serious
health risks, such as atrophy, pigmentation changes, wrinkles,
and malignancy. Ultraviolet rays are epidemiologically and
molecularly related to the three most common types of skin
cancer, basal cell carcinoma, squamous cell carcinoma, and
malignant melanoma. Therefore, it is advisable to carry out this
study, in order to clarify whether there is an association between
exposure to ultraviolet rays and the development of acral
lentiginous melanoma, since it is considered one of the poorly
studied subtypes of melanoma.
Materials and Methods
A narrative review was carried out, in which PubMed, Scielo and Science Direct databases, among others, were searched. The collection and selection of articles was carried out in journals indexed in English from the years 2009 to 2022. As keywords, the following terms were used in the databases according to the DeCS and MeSH methodology: Melanoma; acral; Ultraviolet. In this review, 82 original and review publications related to the subject studied were identified, of which 29 articles met the specified inclusion requirements, such as articles that were in a range not less than the year 2009, that were articles of full text and to report on the relationship between acral lentiginous melanoma and ultraviolet radiation exposure. As exclusion criteria, it was taken into account that the articles did not have sufficient information and that they did not present the full text at the time of review.
Results
Does Ultraviolet Radiation Trigger The Development Of Acral Lentiginous Melanoma?
In order to answer this question, it is advisable to know what its pathophysiological mechanism is, in order to identify the possible sources that could trigger this disease.
Acral lentiginous melanoma, like mucosal melanomas, is a subtype of melanoma with a low mutational load and does not present mutations induced by ultraviolet radiation that are commonly observed in other cutaneous malignant melanomas. Common genetic mutations in Acral Lentiginous Melanoma include KIT, BRAF, NRAS, and NF1.
Specifically, KIT copy number gains are seen in up to 36% of acral melanomas, which is believed to be the most common mutation in Acral Lentiginous Melanoma, which is much higher than in other Cutaneous Malignant Melanomas [13, 14].
In one study, the most commonly identified molecular aberration was chromosomal instability in the cyclin D1 gene and was seen in 45% of Acral Lentiginous Melanoma. Cytological atypical melanocytes surrounding the atypical lesion often harbour KIT copy number gains and cyclin D1 mutations, which is a concept known as “field effect” and may explain local recurrences after lesion excision. Clinic [15-17].
Acral lentiginous melanoma is characterized by a broad radial growth phase in which the melanocytic neoplasm remains in situ. In this phase, single atypical melanocytes increase predominantly at the dermal-epidermal junction in a lentiginous pattern with a propensity to affect adnexal structures.
Pagetoid spread, a common feature of benign and malignant acral melanocytic lesions, may be prominent at the peripheral borders and within the ridges, imparting the characteristic "parallel ridge" dermoscopic findings associated with acral melanocytic neoplasms.
Cytological atypical of melanocytes may be prominent in acral lentiginous melanoma and manifests as nuclear hyperchromasia, enlargement, and variable shape. Invasion typically occurs many years after the lesion begins and consists of nested or single melanocytes invading the dermis. The invasive portion of acral lentiginous melanoma lesions typically resemble superficial spreading melanoma, but may progress to a more nodular morphology over time.
Acral lentiginous melanoma is most commonly an acquired lesion and is associated with a previously existing nevus in less than 11% of cases.
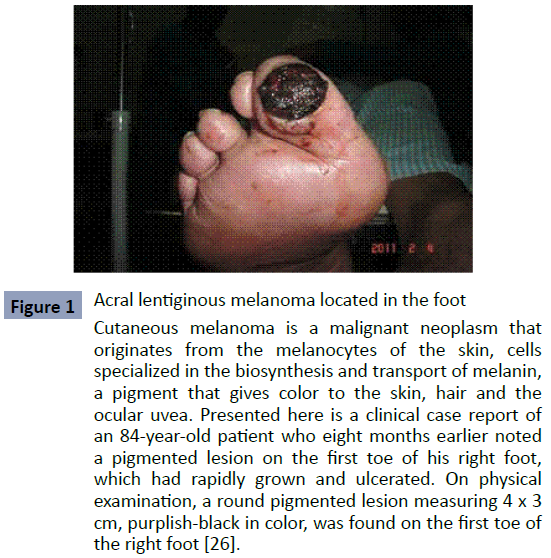
It usually begins as a macule those progresses to a patch with variable light to dark brown pigment. The borders are typically angular, often following the ridges of the dermatoglyphics. As the lesion progresses and invades, it can become nodular and darkly pigmented, blue to black. Ulceration may occur as the lesion becomes more nodular. As we can see in (Figure 1).
Figure 1: Acral lentiginous melanoma located in the foot Cutaneous melanoma is a malignant neoplasm that originates from the melanocytes of the skin, cells specialized in the biosynthesis and transport of melanin, a pigment that gives color to the skin, hair and the ocular uvea. Presented here is a clinical case report of an 84-year-old patient who eight months earlier noted a pigmented lesion on the first toe of his right foot, which had rapidly grown and ulcerated. On physical examination, a round pigmented lesion measuring 4 x 3 cm, purplish-black in color, was found on the first toe of the right foot [26].
Factors Associated With the Development of Acral Lentiginous Melanoma
To date, it is known that multiple studies proposed stress or shear force as a mechanism for the induction of acral lentiginous melanoma, since they were based on association, given that the incidence is greater in the weight-bearing areas of the foot, such as the heel, to the forefoot and the lateral side of the foot [18].
Arch melanoma is rarer, however, it was found to occur more frequently in obese patients. However, more recently, it was found that once corrected for surface area, acral lentiginous melanoma was inversely proportional to atypical acral nevi and benign acral nevi in weight-bearing areas of the foot.
Acral lentiginous melanoma may have a higher incidence on the plantar surfaces compared to the palm of the hand, due to a 50% higher melanocyte density on the sole of the foot.
Furthermore, some correlation has been found in the literature with penetrating injuries increasing the likelihood of acral lentiginous melanoma compared to control patients without penetrating injury.
Typical mutations identified in other types of cutaneous malignant melanoma, which are induced by ultraviolet rays, are not identified in acral lentiginous melanoma. The pathogenesis of acral lentiginous melanoma remains unclear, but genetic factors have been found to contribute.
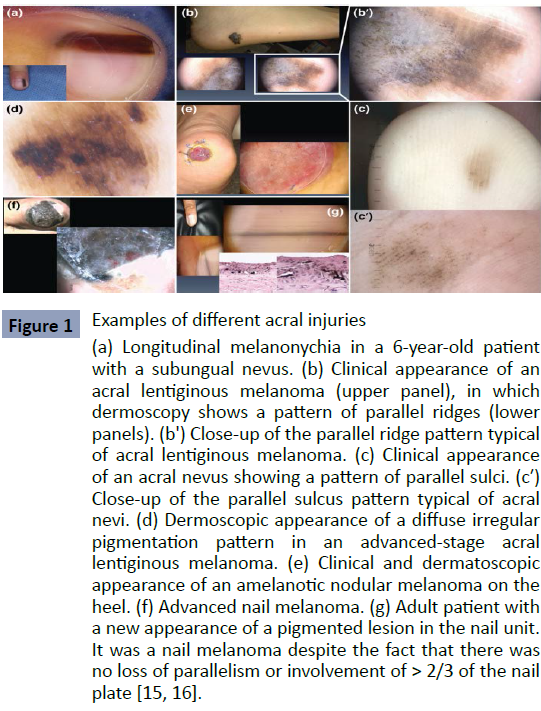
Although subungual, cutaneous, palmar, plantar, etc., lesions tend to be misdiagnosed due to their unusual location and presentation, especially if the doctor is not familiar with this type of melanoma. Therefore, in figure 2 we can show the most frequent forms and locations of acral lentiginous melanoma (Figure 2).
Figure 1: Examples of different acral injuries (a) Longitudinal melanonychia in a 6-year-old patient with a subungual nevus. (b) Clinical appearance of an acral lentiginous melanoma (upper panel), in which dermoscopy shows a pattern of parallel ridges (lower panels). (b') Close-up of the parallel ridge pattern typical of acral lentiginous melanoma. (c) Clinical appearance of an acral nevus showing a pattern of parallel sulci. (c′) Close-up of the parallel sulcus pattern typical of acral nevi. (d) Dermoscopic appearance of a diffuse irregular pigmentation pattern in an advanced-stage acral lentiginous melanoma. (e) Clinical and dermatoscopic appearance of an amelanotic nodular melanoma on the heel. (f) Advanced nail melanoma. (g) Adult patient with a new appearance of a pigmented lesion in the nail unit. It was a nail melanoma despite the fact that there was no loss of parallelism or involvement of > 2/3 of the nail plate [15, 16].
Genetic Mutations Associated With the Development of Acral Lentiginous Melanoma
Elucidating the biological pathways involved in the pathogenesis of acral lentiginous melanoma is key to the development of new molecular-based targeted therapies. Unfortunately, compared to cutaneous melanomas, very few molecular studies have been performed on acral lentiginous melanoma Tumors. Although limited, this body of literature already suggests convincing molecular differences between this disease and other subtypes of melanoma [19].
Sequencing and copy number profiling studies of acral lentiginous melanoma have identified characteristic tumor-promoting mutations involving several genes. Examples of these include the KIT proto-oncogene (KIT), which encodes a type III receptor tyrosine kinase that can stimulate cell growth, division, survival, and migration [20].
Cyclin D1 (CCND1) and cyclin-dependent kinase 4 (CDK4), which encode a cyclin and a kinase that, when bound together, promote the transition from G1 to S phase; cyclin-dependent kinase inhibitor 2A ( CDKN2A ), which encodes two proteins: p16(INK4A) and p14(ARF), the latter of which helps stabilize TP53; telomerase (TERT), encodes an enzyme crucial for telomere maintenance and aurora kinase A (AURKA), which is involved in regulating cell cycle progression by playing a critical role in mitosis.
Identification of mutational signatures from a collection of cancer genomes can identify the processes, both environmental and endogenous, that operated during tumor development and consequently caused the accumulation of mutations. As expected, several analyses of mutational signatures in cutaneous melanoma have identified a high prevalence of the UV-induced signature, but not in association with acral lentiginous melanoma.
Discussion
The study conducted by [21], showed that the incidence of
acral lentiginous melanoma in the United States has remained
relatively stable over time, unlike cutaneous malignant
melanoma in general, where the incidence has been steadily
increasing. During the 1970s, the incidence rate of cutaneous
malignant melanoma increased rapidly by approximately 6%
per year. Since 1981, the rate of increase has slowed to 1 to 3%
per year. (27) The steady increase in the incidence of cutaneous
malignant melanoma is likely due to increased ultraviolet radiation, although increased surveillance, physician and patient
education, and sun safety measures have dramatically reduced
the rate. of increase. Although, as demonstrated in this study,
there is no direct relationship between ultraviolet radiation
exposure and the development of acral lentiginous melanoma, it
is therefore necessary to investigate more about this pathology,
either individually or in association with melanoma. Cutaneous
malignant. [22-28].
Another study by Phan A, et al, demonstrated that melanoma in
people of color has a predilection for acral locations, especially
in the plantar regions. This also appears to be true in that the
most common locations for acral lentiginous melanoma were
the lower extremities in all racial groups. (29) This predilection
for the development of acral lentiginous melanoma in plantar
locations has led many to believe that trauma may be important
in the etiology of this disease, since sun exposure has not been
shown to be a risk factor. For their development. [18, 29]
Strength of the current study is the implemented methodology,
regarding the literature search, and steps in the selection
of relevant articles, quality assessment and data extraction.
However, this study has several limitations, which should be
taken into account before reaching a conclusion, among these are
the little evidence from the analysis of clinical trials to determine
with certainty the cause of the development of acral lentiginous
melanoma, or if there is a relationship between the development of this disease due to exposure to ultraviolet radiation, so more
studies are needed to answer these questions.
Conclusion
Acral lentiginous melanoma, like mucosal melanomas, is a subtype of melanoma with a low mutational load and does not present mutations induced by ultraviolet radiation that are commonly observed in other cutaneous malignant melanomas.
Acral lentiginous melanoma is characterized by a broad radial growth phase in which the melanocytic neoplasm remains in situ. In this phase, single atypical melanocytes increase predominantly at the dermal-epidermal junction in a lentiginous pattern with a propensity to affect adnexal structures. Acral lentiginous melanoma is most commonly an acquired lesion and is associated with a previously existing nevus in less than 11% of cases.
To date, it is known that multiple studies have proposed stress or shear force as a mechanism for the induction of acral lentiginous melanoma, given that the incidence is higher in weight-bearing areas of the foot, such as the heel, forefoot and ball of the foot. lateral side of the foot.
Sequencing and copy number profiling studies of acral lentiginous melanoma have identified characteristic tumor-promoting mutations involving several genes. Among the most common in Acral Lentiginous Melanoma include KIT, BRAF, NRAS and NF1.`
REFERENCES
- Huang K, Fan J, Misra S (2020) Acral Lentiginous Melanoma: Incidence and Survival in the United States, 2006-2015, an Analysis of the SEER Registry. J Surg Res 251:329-339.
Indexed at, Google Scholar, Crossref
- Fernandez-Flores A, Cassarino DS (2017) Histopathological diagnosis of acral lentiginous melanoma in early stages. Ann Diagn Pathol 26:64-69.
Indexed at, Google Scholar, Crossref
- Jung HJ, Kweon SS, Lee JB, Lee SC, Yun SJ (2013) A clinicopathologic analysis of 177 acral melanomas in Koreans: relevance of spreading pattern and physical stress. JAMA Dermatol 149:1281-1288.
Indexed at, Google Scholar, Crossref
- Minagawa A, Omodaka T, Okuyama R (2016) Melanomas and Mechanical Stress Points on the Plantar Surface of the Foot. N Engl J Med 374:2404-2406.
Indexed at, Google Scholar, Crossref
- Sheen YS, Liao YH, Lin MH, Chen JS, Liau JY (2017) A clinicopathological analysis of 153 acral melanomas and the relevance of mechanical stress. Sci Rep 7:5564.
Indexed at, Google Scholar, Crossref
- Costello CM, Pittelkow MR, Mangold AR (2017) Acral Melanoma and Mechanical Stress on the Plantar Surface of the Foot. N Engl J Med 377:395-396.
Indexed at, Google Scholar, Crossref
- Ghanavatian S, Costello CM, Buras MR, Cumsky HJL, Pittelkow MR (2019) Density and distribution of acral melanocytic nevi and acral melanomas on the plantar surface of the foot. J Am Acad Dermatol. 80:790-792.e2.
Google Scholar, Crossref
- Lino-Silva LS, Dominguez-Rodriguez JA, Aguilar-Romero JM, Martinez-Said H, Salcedo-Hernández RA (2016) Melanoma in Mexico: Clinicopathologic Features in a Population with Predominance of Acral Lentiginous Subtype. Ann Surg Oncol 23:4189-4194.
Indexed at, Google Scholar, Crossref
- Wei X, Wu D, Li H, Zhang R, Chen Y (2020) The Clinicopathological and Survival Profiles Comparison Across Primary Sites in Acral Melanoma. Ann Surg Oncol 27:3478-3485.
Indexed at, Google Scholar, Crossref
- Merkel EA, Gerami P (2017) Malignant melanoma of sun-protected sites: a review of clinical, histological, and molecular features. Lab Invest 97:630-635.
Indexed at, Google Scholar, Crossref
- Darmawan CC, Jo G, Montenegro SE, Kwak Y, Cheol L et al. (2019) Early detection of acral melanoma: A review of clinical, dermoscopic, histopathologic, and molecular characteristics. J Am Acad Dermatol 81:805-812.
Indexed at, Google Scholar, Crossref
- D John J, Stuart A, Alexandra S (2013) Timothy UV Radiation and the Skin. Int J Mol Sci 14:12222–12248.
Indexed at, Google Scholar, Crossref
- Bianca M, Johann W, Anne M, Willem I, Maritha J et al. (2020) The tumor genetics of acral melanoma: What should a dermatologist know? JAAD Int 1:135-147.
Indexed at, Google Scholar, Crossref
- M Ryung, K Jihyun, Y Kee (2010) Treatment and Outcomes of Melanoma in Acral Location in Korean Patients. Yonsei Med J 51:562-568.
Indexed at, Google Scholar, Crossref
- Mariachiara A, Cristina Z, Simone C, Chiara R (2018) Sun Exposure and Melanoma, Certainties and Weakness of the Present Knowledge. Front Med 5:235.
Google Scholar, Crossref
- Asta J, Zivile B, Johan M (2014) Sun exposure and melanomas on sun-shielded and sun-exposed body areas. Adv Exp Med Biol 810:375-89.
Indexed at, Google Scholar, Crossref
- Robert V, Peter A, Nicholas K, Nicola W, Ann M (2017) Unexpected UVR and non-UVR mutation burden in some acral and cutaneous melanomas. Lab Invest 97:130-145.
Indexed at, Google Scholar, Crossref
- Gretchen M, Vito W (2021) Emerging strategies to treat rare and intractable subtypes of melanoma. Pigment Cell Melanoma Res 34:44-58.
Indexed at, Google Scholar, Crossref
- Brett C, Taylor G, Maheera F, Richard M (2020) Acral Lentiginous Melanoma: A Rare Variante With Unique Diagnostic Challenges. Cureus 12:e8424.
Indexed at, Google Scholar, Crossref
- Krishnaraj M, Komal S, Christine S, Georgia J (2010) Malignant Melanoma in African–Americans.
Indexed at, Google Scholar, Crossref
- A Population-Based Clinical Outcomes Study Involving 1106 African-American Patients from the Surveillance, Epidemiology, and End Result (SEER) Database (1988–2011). Medicine (Baltimore). 96:e6258.
Google Scholar, Crossref
- Meg W, Dawn M, Maryellen M (2016) Ultraviolet Radiation Exposure and It Impact on Skin Cancer Risk. Semin Oncol Nurs 32:241-254.
Indexed at, Google Scholar, Crossref
- Jennifer A, David E (2014) The melanoma revolution: from UV carcinogenesis to a new era in therapeutics. Science 346:945-949.
Indexed at, Google Scholar, Crossref
- Ashley S, Yu-Ying H (2018) Mechanism and prevention of UV-induced melanoma. Photodermatol Photoimmunol Photomed 34:13-24.
Google Scholar, Crossref
- Sang Y, Sook J (2016) Cutaneous Melanoma in Asians. Chonnam Med J 52:185-193.
Indexed at, Google Scholar, Crossref
- Jose R, Eduardo C, Ubaldo V (2014) Melanoma lentiginoso acral: a proposito de un caso. Acral lentiginous melanoma: a case report. Medicent Electron 18.
Indexed at, Google Scholar
- Porcia T, Alisa M, Mary L, Margaret A (2009) Acral Lentiginous Melanoma: Incidence and Survival Patterns in the United States, 1986-2005. Arch Dermatol 145:4270-434.
Indexed at, Google Scholar, Crossref
- Patricia B, Christia M, Carolina C, Martha V, Omar G (2021) Acral lentiginous melanoma: Basic facts, biological characteristics and research perspectives of an understudied disease. Pigment Cell Melanoma Res 34:59-71.
Indexed at, Google Scholar, Crossref
- Phan A, Touzet S, Dalle S, Ronger-Savle S, Balme B et al. (2006) Acral lentiginous melanoma: a clinicoprognostic study of 126 cases 155:561-569.
Indexed at, Google Scholar, Crossref
Citation: GarcIa Medina LC, Gamero Tafur
MP, Marin Murillo MC, Ruiz Atencio YY,
Osorio Burgos RA, et al. (2022) Relationship
between Acral Lentiginous Melanoma and
Exposure to UV Radiation. Health Sci J. Vol.
16 No. S7: 945.