Review Article - (2022) Volume 16, Issue 12
Significance of SARS-CoV-2 and Influenza A+B Antigen Combo Rapid Test for Distinguishing Influenza from COVID-19
Zhang Lei1*,
Yang Feng2 and
WuYanli2
1Zhejiang Gongshang University, China
2Community Health Service Center, Yipeng Street, Qiantang District,Hangzhou, China
*Correspondence:
Zhang Lei, Zhejiang Gongshang University,
China,
Email:
Received: 30-Nov-2022, Manuscript No. Iphsj-22-13221;
Editor assigned: 02-Dec-2022, Pre QC No. Iphsj-22-13221(PQ);
Reviewed: 16-Dec-2022, QC No. Iphsj-22-13221;
Revised: 21-Dec-2022, Manuscript No. Iphsj-22-13221(R);
Published:
28-Dec-2022, DOI: 10.36648/1791- 809X.16.12.986
Abstract
Both seasonal influenza viruses (including influenza A and B viruses) and COVID-19 are infectious viruses that cause respiratory illness.
According to the Centers for Disease Control (CDC), typical flu symptoms include fever, cough, sore throat, muscle aches, headache, runny or stuffy nose, fatigue, and sometimes even vomiting and diarrhea [1]. Flu symptoms usually appear suddenly.Most people who get the flu recover within two weeks. But in some people, the flu can cause complications, such as pneumonia. So far this flu season, about 1 percent of Americans have developed symptoms severe enough to require hospitalization, similar to the previous season's incidence.
For COVID-19, the 38th WHO regulatory update on COVID-19 mentions that more than 220 million confirmed cases and 4.5 million deaths of COVID-19 have been reported to WHO globally. The situation varies from region to region. Some regions and countries continue to see dramatic increases in the number of cases and deaths, while others are declining.
Vaccinations, physical proximity, hand washing, avoiding crowded and enclosed spaces, and wearing masks are all "anti-blockade measures": they can prevent the spread of disease without having to shut down large parts of society.
The WHO has said it is important to note that it is difficult to distinguish between different respiratory viruses based on symptoms alone because respiratory viruses can cause similar symptoms. However, the treatment options for COVID-19 and influenza viruses are very different. To differentiate and facilitate follow-up treatment to treat the symptoms, the rapid antigen test product can be used to obtain a relatively accurate result in a few minutes with a simple operation that can be used for initial screening purposes. This report evaluated the performance of the AllTest SARS-CoV-2 and Influenza A+B Antigen Combo Rapid Test (Nasal Swab) and discuss the importance of differentiating COVID-19 from influenza.
Keywords
Influenza; SARS-Cov-2; Antigen Test; Nasal Swab
INTRODUCTION
About Influenza
Influenza is an acute viral respiratory disease caused by infection of the respiratory tract with influenza viruses (seasonal influenza A and B viruses) that circulate among people worldwide. Seasonal influenza refers to a disease in humans caused by infection with seasonal influenza A or B viruses. Annual influenza epidemics of variable severity typically occur during colder periods in temperate climates worldwide [1].
Year-round influenza activity can be observed in tropical and subtropical areas, peaking at different times [2]. Most people with influenza have self-limited upper-respiratory-tract symptoms with or without systemic signs and symptoms that temporarily affect daily activities, including missing work or school, and some might access medical care. Some individuals with influenza, particularly young children, older adults, pregnant people, and those with certain underlying conditions, can have complications resulting in medical care visits, hospital admissions, or in-hospital and community deaths [3].
Difference between COVID-19 and Influenza
COVID-19 and Influenza are both infectious respiratory diseases, but they are caused by different viruses. COVID-19 is caused by a coronavirus (SARS-CoV-2) infection that was first identified in 2019. Influenza is caused by influenza viral infections.
COVID-19 is more easily transmitted than influenza. Efforts to maximize the proportion of people vaccinated against COVID-19 remain critical to reducing the risk of serious COVID-19 illnesses and deaths.
COVID-19 can cause more severe diseases in some people compared to influenza. People infected with COVID-19 may take longer to develop symptoms and contagion may last longer than in people with influenza.
We cannot distinguish influenza from COVID-19 by symptoms alone because they share some of the same signs and symptoms (Table 1).
| Signs and Symptoms |
Influenza |
COVID-19 (at least two) |
| Symptom onset |
Sudden |
Gradual |
| Fever |
Temperature of 100°F and above lasting 3-4 days |
Temperature of 100°F and above lasting 2-7 days |
| Chills |
Common |
Common |
| Headache |
Prominent |
Common |
| Cough |
Dry, sometimes severe |
Dry, persistent, and often more severe |
| Sore throat |
Sometimes |
Common, and often prominent |
| Runny or stuffy nose |
Sometimes |
Common |
| Shortness of breath |
Sometimes |
Common, and often prominent |
| Muscle pain/body aches |
Usual, often severe |
Common |
| Repeated shaking with chills |
Rare |
Sometimes |
| Fatigue |
Early and prominent |
Common |
| Diarrhea |
Sometimes (more common in children) |
Sometimes (more common in children) |
| Vomiting or nausea |
Sometimes (more common in children) |
Sometimes (more common in children) |
| Sudden loss of taste/smell |
Never |
Common |
Table 1.Comparing COVID-19 and Flu Symptoms.
Specialized testing is needed to determine the correct disease, and having a medical professional perform a specific test that detects influenza and COVID-19 can lead to faster diagnosis and treatment for the specific virus. Early treatment of COVID-19 and influenza can reduce the risk of developing serious illness. Testing can also reveal whether a person has both the flu and COVID-19, although this is not common. People who have both the flu and COVID-19 may be sicker than people who have the flu or COVID-19 alone [9]. In addition, some people with COVID-19 may also be affected by a post-COVID state (also known as long COVID).
Prevention Methods and Treatment for Flu and COVID-19
For Flu: If you have the flu, antiviral medications may be a treatment option. Antiviral medications work best when taken early, such as 1-2 days after flu symptoms appear.
If you are at higher risk for serious flu complications and develop flu symptoms, contact your healthcare provider. People at higher risk of flu complications include children, adults 65 years of age and older, pregnant people, and people with specific medical conditions such as asthma, diabetes, and heart disease.
When treatment is started within 1-2 days of the onset of flu symptoms, flu antiviral medications can reduce symptoms and shorten the duration of illness by 1-2 days. They can also prevent some flu complications, such as pneumonia. For people at higher risk for serious flu complications, treatment with flu antivirals may mean a milder or more serious illness that may require hospitalization [6].
For COVID-19: The risk of SARS-CoV-2 transmission can be reduced by covering one’s mouth when coughing and sneezing and keeping at least 6 feet away from others. When consistent distance is not possible, masks may reduce the spread of infectious droplets from SARS-CoV-2-infected individuals. Frequent hand washing is also effective in reducing the risk of infection [4]. Healthcare providers should follow the Centers for Disease Control and Prevention (CDC) recommendations for infection control and the appropriate use of personal protective equipment [5].
As for the COVID-19 treatment, the FDA has approved certain antiviral drugs and monoclonal antibodies for the treatment of patients with mild to moderate COVID-19 who are more severely ill.
Antiviral treatments target specific parts of the virus, stopping it from multiplying in the body and helping to prevent serious illness and death. Monoclonal antibodies help the immune system recognize and respond more effectively to the virus.
Significance of Detecting Respiratory Disease Types
Respiratory diseases and disorders affect the respiratory tract and lungs involved in breathing.
Influenza and Para influenza viruses can cause serious illness. Influenza viruses attack many systems of the body simultaneously, but the primary site of virus replication appears to be the alveolar cells of the lungs. There, the virus multiplies exponentially within 24 hours and can involve the lungs starting from the lung parenchyma, causing severe solid changes and inflammation of the lung tissue. Severe tracheitis, bronchiectasis and fine bronchiectasis often occur together. Another form of the disease, known as viral pneumonia, is distinguished by the presence of patchy pulmonary atelectasis or partial collapse of lung tissue without extensive involvement of the bronchial tree. The lungs are the site of severe episodes of influenza, which may occur rapidly with secondary infections. All these conditions are more dangerous in young children and the elderly.
The effects of COVID-19 on the human body are well known, and the damage caused by this disease is not only irreversible, but also has sequelae including Long COVID. That is why it is important to prevent and treat respiratory diseases, no matter what they are [6]. Because some of the symptoms of influenza and COVID-19 are similar, it may be difficult to distinguish between the two respiratory diseases based on symptoms alone. Therefore, it is necessary to test for both viruses in anyone with symptoms of COVID-19 or influenza. Testing can be performed using mainstream methods including nucleic acid testing or by the SARS-CoV-2 antigen assay [7, 8].
Comparison of RT-PCR Method to Evaluate the Performance of AllTest SARS-CoV-2 and Influenza A+B Antigen Combo Rapid Test (Nasal Swab)
Materials and Directions for Use
The All Test SARS-CoV-2 and influenza A+B antigen combo rapid test kit includes a package insert, sterile swab, extraction buffer, and an optional biosafety bag.
The SARS-CoV-2 and Influenza A+B Antigen Combo Rapid Test (Nasal Swab) is a qualitative membrane-based immunoassay for the detection of SARS-CoV-2 nucleocapsid protein, Influenza A and Influenza B nucleoproteins antigens in human swab specimen.
Before testing, wash your hands with soap and water for at least 20 seconds before and after the test. If soap and water are not available, use hand sanitizer with at least 60% alcohol [9].
Testing immediately after opening the aluminium foil pouch gives the most accurate test results.
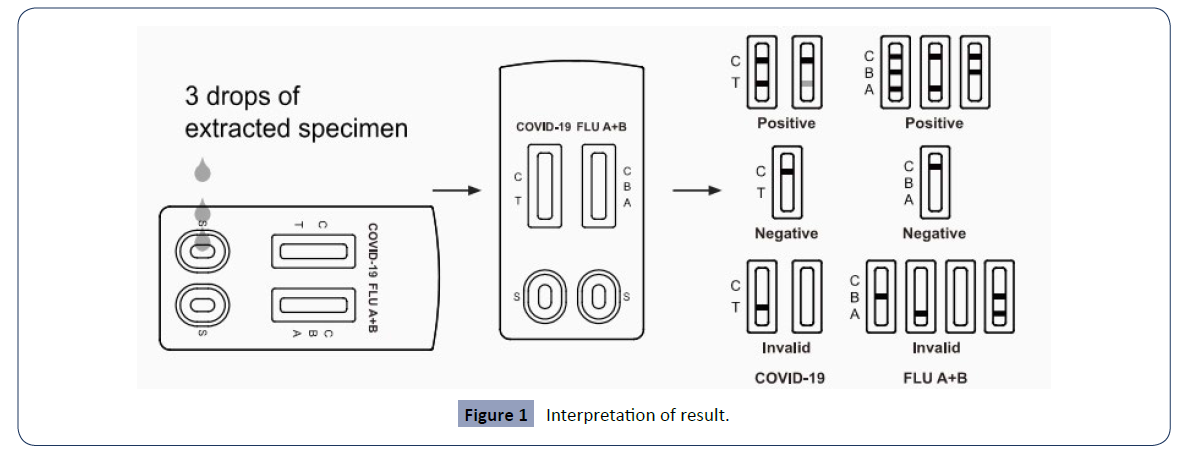
Place the test cassette on a flat, level surface. Turn the sample extraction tube upside down, add 3 drops of extracted sample to each sample well(s) of the test cassette and start the timer and read the results (see Figure 1.) after ten minutes and no more than 20 minutes.
Finally, after the test is completed, place all the components into the plastic bag and tightly seal and dispose of according to local regulations (Figure 1).
Figure 1: Interpretation of result..
The test results are used to detect SARS-CoV-2 nucleocapsid protein, Influenza A virus and Influenza B virus nucleoprotein antigens. During the acute phase of infection, antigens are typically detected in upper respiratory specimens. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is required to determine infection status.
Positive results indicate the presence of SARS-CoV-2 and/or influenza A+B. Individuals who test positive should self-isolate and seek additional care from a healthcare provider. A positive result does not exclude bacterial infection or co-infection with other viruses. A negative result does not rule out SARS-CoV-2 and/or influenza A+B infection. Individuals who test negative and continue to have COVID-19-like or influenza-like symptoms should seek follow-up care from a healthcare provider.
Clinical Performance of AllTest SARS-CoV-2 and Influenza A+B Antigen Combo Rapid Test (Nasal Swab)
The SARS-CoV-2 and Influenza A+B Antigen Combo Rapid Test (Nasal Swab) has been evaluated with specimens obtained from patients. RT-PCR is used as the reference method for the SARSCoV- 2 and Influenza A+B Antigen Combo Rapid Test (Nasal Swab). Specimens were considered positive if RT-PCR indicated a positive result. Specimens were considered negative if RT-PCR indicated a negative result (Table 2, Table 3).
| SARS-CoV-2 and Influenza A+B Antigen Combo Rapid Test |
RT-PCR (nasopharyngeal swab) |
Total |
| Positive |
Negative |
SARS-CoV-2
Antigen |
Positive |
161 |
2 |
163 |
| Negative |
5 |
482 |
487 |
| Total |
166 |
484 |
650 |
| Relative Sensitivity |
96.99% (95%CI: 93.11%~99.01%) |
| Relative Specificity |
99.59% (95%CI: 98.52%~99.95%) |
| Accuracy |
98.92% (95%CI: 97.79%~99.57%) |
Table 2. Performance Characteristics of SARS-CoV-2 Test.
Relative sensitivity: 96.99% (95%CI: 93.11%~99.01%)
Relative specificity: 99.59% (95%CI: 98.52%~99.95%)
Accuracy: 98.92% (95%CI: 97.79%~99.57%)
| SARS-CoV-2 and Influenza A+B Antigen Combo Rapid Test |
RT-PCR (nasopharyngeal swab) |
Total |
| Positive |
Negative |
Influenza A
Antigen |
Positive |
68 |
2 |
70 |
| Negative |
3 |
485 |
488 |
| Total |
71 |
487 |
558 |
| Relative Sensitivity |
95.77% ï¼?95%CI: 88.14%~99.12%ï¼? |
| Relative Specificity |
99.59% ï¼?95%CI: 98.52%~99.95%ï¼? |
| Accuracy |
99.10% (95%CI: 97.92%~99.71%) |
| SARS-CoV-2 and Influenza A+B Antigen Combo Rapid Test |
RT-PCR (nasopharyngeal swab) |
Total |
| Positive |
Negative |
Influenza B
Antigen |
Positive |
48 |
3 |
51 |
| Negative |
3 |
504 |
507 |
| Total |
51 |
507 |
558 |
| Relative Sensitivity |
94.12% (95%CI: 83.76%~98.77%) |
| Relative Specificity |
99.41% (95%CI: 98.28%~99.88%) |
| Accuracy |
98.92% (95%CI: 97.67%~99.60%) |
Table 3. Performance Characteristics of Influenza A+B Test.
Influenza A Antigen Test
Relative sensitivity: 95.77% (95%CI: 88.14%~99.12%)
Relative specificity: 99.59% (95%CI: 98.52%~99.95%)
Accuracy: 99.10% (95%CI: 97.92%~99.71%) Influenza B Antigen Test
Relative sensitivity: 94.12% (95%CI: 83.76%~98.77%)
Relative specificity: 99.41% (95%CI: 98.28%~99.88%)
Accuracy: 98.92% (95%CI: 97.67%~99.60%)
Summary
The above comparison experiments with the RT-PCR method showed that AllTest SARS-CoV-2 and Influenza A+B Antigen Combo Rapid Test (Nasal Swab) performed well and showed high standards of product quality in terms of specificity, sensitivity, and accuracy. It is worth noting the product can be tested for two viruses through a single specimen collection, which greatly improves the efficiency of testing and reduces the discomfort and inconvenience caused by specimen collection.
The results of the tested samples show that SARS-CoV-2 and Influenza A+B Antigen Combo Rapid Test (Nasal Swab) developed by AllTest meets the requirements of the intended use for professional in vitro diagnostics. Thus, this combo rapid test kit can be used to distinguish influenza from COVID-19.
REFERENCES
- Centers for Disease Control and Prevention (2021). Flu Symptoms & Diagnosis.
Google Scholar
- Azziz Baumgartner E, Dao CN, Nasreen S (2012) Seasonality timing and climate drivers of influenza activity worldwide. J Infect Dis 206: 838-846.
Indexed at,Google Scholar, Crossref
- Paules C, Subbarao K (2017) Influenza Lancet 390: 697-708.
Google Scholar
- Centers for Disease Control and Prevention. How to protect yourself and others (2022).
Google Scholar
- Centers for Disease Control and Prevention. Infection control guidance for healthcare professionals about coronavirus (COVID-19). 2020.
Google Scholar
- Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD).
Google Scholar
- FDA Individual EUAs for Antigen Diagnostic Tests for SARS-CoV-2 Note: Because antigen detection assays have lower sensitivity than nucleic acid detection assays, a negative result does not exclude SARS-CoV-2 infection and should be confirmed by nucleic acid detection assay.
Google Scholar
- CDC FDA-cleared Nucleic Acid Detection Based Tests for Influenza Viruses.
- CDC. Similarities and Differences between Flu and COVID-19
Citation: Lei Z, Feng Y, Yanli W (2022) Significance of SARS-CoV-2 and Influenza A+B Antigen Combo Rapid Test for Distinguishing Influenza from COVID-19. Health Sci J. Vol. 16 No. 12: 986.