Luiz Fernando Zmetek Granja2*, Lysianne Pinto2, Jaqueline Souza Silvestre1, Cátia Amancio Almeida1, Daniela Sales Alviano1, Galba Maria Campos Takaki3, Maria Helena da Silva2, Regina Ejzemberg2 and Celuta Sales Alviano1
1Department of General Microbiology, Center for Research in Ambienais Sciences, Catholic University of Pernambuco, Recife , PE, Brazil
2Department of Immunology, Center for Research in Ambienais Sciences, Catholic University of Pernambuco, Recife , PE, Brazil
3Department of Chemistry, Center for Research in Ambienais Sciences, Catholic University of Pernambuco, Recife , PE, Brazil
*Corresponding Author:
Luiz Fernando Zmetek Granja
Department of Immunology, I2-065 room
Institute of Microbiology Professor Paulo de Goes
Federal University of Rio de Janeiro, Av Carlos Chagas Filho 373
Rio de Janeiro, RJ, CEP: 21941-902, Brazil
Tel: +55-021-2560-8344
Email: lfzmetek@gmail.com
Received date: October 28, 2015 Accepted date: January 05, 2016 Published date: January15, 2016
Copyright: © 2016, Granja LFZ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Complement system; Fungi; Mucor polymorphosporus; Spores; Yeasts; Zygomycetes
Introduction
Mucormycosis is a severe disease that may affect susceptible people after intake [1] or inhalation [2] of spores from fungi of the Mucorales order [3]. Usually, cutaneous, pulmonary, rhinocerebral, gastrointestinal and disseminated outcomes are common manifestations for the disease [4]. Diabetes and its acidosis, neutropenia, leukemia and lymphoma have all been associated with the occurrence of this disease, indicating that it is usually opportunistic in nature [5]. Spores from these fungi usually give place to hyphae which is mainly responsible for tissue damage [6]. Even though that is true, yeasts from the Mucorales order have been reported in urine from a patient with bladder infection [7].
While Rhizopus sp is accountable for the majority of mucormycosis infections [3], a large number of cases were reported with other species from the Mucor genus as the causes of infection [8-11].
The complement system is a complex and extensively regulated system, which contains both proteins that are soluble or cell bound and that can be activated, producing diverse outcomes such as: opsonization (C3b; C4b) and/or lysis (C5b-C9) of vulnerable microbes [12]. The activation may be triggered by three different pathways: one normally dependent on antigen-antibody reactions, the classical pathway; another initiated by spontaneous hydrolysis of the C3 thioester bond, the alternative pathway; and the lectin pathway, that uses Mannan Binding Lectin (MBL) or ficolins which recognizes certain carbohydrate patterns on the surface of microbes [13].
On a previous work [14], we displayed by ELISA the presence of C3, C4, MBL and IgG on spores of M. circinelloides, M. ramosissimus and M. plumbeus after complement activation. All species tested presented similar results. Considering this data, the major purpose of this work was to investigate if the same would occur on M. polymorphosporus spores and yeasts.
Materials and Methods
Fungal strain: Mucor polymorphosporus 1044 is a clinical specimen from Colecao de Culturas of Micoteca University Recife Mycologia (URM). URM is located at Universidade Federal De Pernambuco (UFPE), Brazil. For the experiments the fungus was cultured in plates containing Sabourauddextrose medium for 5 days at room temperature. Spores were harvested from the media in PBS pH 7.0. The fungus was also cultured, in Sabouraud-dextrose containing plates, in 30% CO2 atmosphere using GasPak jars for 48 hours at 37°C. Yeasts were washed twice with PBS pH 7.0 Spore and yeast concentrations were determined by counting in Neubauer chamber [7,15].
Complement source: A pool of Normal Human Serum (NHS) was obtained after 10 ml of blood was drawn from each of our coworkers [6], with their previous consent. The pool was adsorbed three times, each time with a pellet of sheep erythrocytes (109 cells ml-1 final concentration) for 30 minutes at 4°C, to remove any possible reacting antibodies against sheep erythrocytes. After centrifugation at the same temperature, supernatant was collected, aliquoted and kept at –80°C.
Hemolytic system: Sheep blood was drawn in Alsever’s solution and then a sample was centrifuged (1400xg). The supernatant was removed and the pellet was washed twice with PBS pH 7.0. The pellet was then suspended in veronal buffered saline (VBS - 0.1% gelatin, 5mM sodium Veronal, 142 mM NaCl pH 7.35) containing Ca2+ (1.5 × 10-4M) and Mg2+ (1 × 10-3M) in order to contain 109 cells ml-1. To this suspension, we added equal volume of rabbit anti-sheep erythrocytes antibodies (Sigma Chemical Co. USA) diluted in VBS (1/3200), as previously described by Lima & Silva [16]. The mixture was incubated 30 minutes at 37°C. The suspension was then adjusted to 5 × 108 cells ml-1 according to Mayer [17].
Complement activators: The following were used in complement activation experiments: Spores and yeasts of M. polymorphosporus; Zymosan A (Sigma Chemical Co. USA), which was employed as the activation control of the alternative pathway.
Complement activation: M. polymorphosporus spores (108 cells ml-1) and yeasts (107 cells ml-1) were incubated with human adsorbed serum, treated or not with chelators (either 10 mM EDTA or 10 mM EGTA with 5 mM MgCl2) at 37°C for 60 minutes. The final dilution of the serum was 1/10 in VBS (with chelators) or VBS with Ca2+ and Mg2+ (as described above). Serum treated or not with chelators without activators was used as negative control and 1 mg ml-1 of Zymosan was used as positive control. After incubation, the samples were centrifuged (1400xg) at 4°C. After activation, the sera were diluted 1/5 in VBS with Ca2+ and Mg2+, making the final dilution of the sera 1/50. The residual complement was quantified in the resulting sera using the hemolytic system [17]. Briefly, the antibody coated-sheep erythrocytes is placed in contact with increasing volumes of this diluted serum (1/50) [17], which allows complement activation by the classical pathway, if there is still enough complement components in the sera, after complement activation with our samples. The amount of hemoglobin released was determined in a spectrophotometer (Beckmann) at λ 540 nm. CH50 ml-1 was calculated by von Krogh’s equation [17] and consumption percentages were determined according to positive and negative controls values.
ELISA: To assess complement fragments (C3, C4), CRP, IgG and MBL deposited on the surface of samples, 96 wells polystyrene plates (Corning, USA) were coated with 100 μL of 107 spores in PBS pH 7.0 or 106 yeasts in PBS pH 7.0 obtained from complement activation (106 spores or 105 yeasts per well). The plates were incubated for 1h/37°C and then overnight at 4°C. After washing with PBS pH 7.0, blocking buffer (2% BSA in PBS) was added and incubated for two hours at 37°C. In order to detect C3 fragments, goat antihuman C3 antibody, diluted 1/3000 (Calbiochem. USA) was added. To detect C4 fragments, rabbit antihuman C4, at 1/400 (DAKO immunoglobulins A/S. Denmark) was used. To detect IgG, goat antihuman IgG, 1/5000 (Santa Cruz Biotechnology Inc. EUA). For CRP, rabbit antihuman CRP, 1/50 (Santa Cruz Biotechnology Inc. EUA) was included. For MBL, rabbit antihuman MBL, 1/50 (Santa Cruz Biotechnology Inc. EUA) was used. The plates were incubated for 1h/37°C. After incubation, the plates were washed 3 times with PBS pH 7.0. Secondary antibodies conjugated with peroxidase were then added to react for 60 minutes at 37°C. The terminal complexes were detected after incubation for 20 minutes with substrate solution pH 5.0 (4 μl H2O2 and 4 μg OPD in 10 ml of 100 mM citric acid, 100 mM NaH2PO4). The absorbance was measured at 490 nm (SLTSpectra, Austria) after the reaction was stopped with 2N H2SO4 [18]. C3 and C4 detection were assayed using fungi incubated with serum treated or not with chelators, while IgG, CRP and MBL only used fungi incubated with serum without chelators. Non-activated samples, which had not been incubated with serum, were used in all tests as negative controls.
Immunofluorescence assay: The pellets resultant from the activation were washed 3 times each with PBS pH 7.0 and resuspended in 1ml of the same buffer. 10 μL samples of the fungi were distributed in immunofluorescence slides. After heat fixation, the slides were treated with 10 μL of fluorescein conjugated rabbit anti-human C3c (DAKO-immunoglobulins A/S. Denmark), placed at 37°C for 1 hour and then washed with PBS. Samples were counterstained with 0.1% Evan’s blue dye [19], to suppress auto-fluorescence. The slides were microscopically observed on an Axioplan 2 epi-fluorescence microscope (Zeiss, Germany).
Statistical methods: Complement activation, ELISA and immunofluorescence assays were repeated three times. Complement consumption mean values were taken into account for evaluation. Analysis of variance and two tailed two sample t-test were applied to compare results from each test.
Results
Complement activation
The M. polymorphosporus forms were studied on their capacity to activate the human complement system in vitro, using adsorbed human serum with or without chelators (EDTA or EGTA). Chelator presence on the serum had the purpose of checking the activation pathway utilized and potential cleavage of components by Ca2+ and/or Mg2+ independent enzymes of fungal origin [20]. The results can be observed in Figure 1. When samples were incubated with EDTA, no reduction in hemolytic activity was detected, while adding EGTA-Mg2+, which elicits only the alternative pathway, or the lack of any of these substances, which, in turn, permits all pathways, confirmed full complement consumption. The serum without the presence of any activating particle (negative control) did not lose any hemolytic activity, even if there was addition of chelators. Both forms examined demonstrated the similar result pattern (p > 0.05).
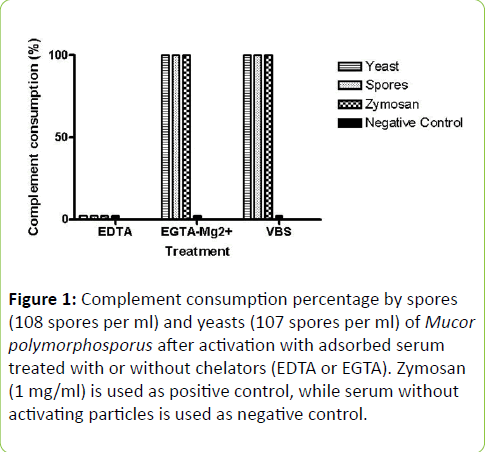
Figure 1: Complement consumption percentage by spores (108 spores per ml) and yeasts (107 spores per ml) of Mucor polymorphosporus after activation with adsorbed serum treated with or without chelators (EDTA or EGTA). Zymosan (1 mg/ml) is used as positive control, while serum without activating particles is used as negative control.
C3 and C4 fragments detection on fungal surfaces
C3 and C4 fragments deposited onto samples subsequent to activation, was assessed by ELISA. Both forms tested bound to C3 fragments in a similar manner (Figure 2). The data indicates efficient alternative pathway activation, since without chelators, the results were only a little superior than with EGTA-Mg2+ (p < 0.05). When comparing spore and yeast forms, the results overall showed that a higher amount of C3 bound to spores (p < 0.05) rather than yeasts (Fig. 2). C4 fragments results were quite contrasting (p < 0.001) for both forms, particularly when tested without any chelators (Figure 2), which corroborates with complement activation by the classical pathway. The amount of C4 which bound to spores was over 2 folds higher than on yeasts. C3 results for zimosan were higher than the other species evaluated (p < 0.001), when testing occurred either with or without EGTA. Zymosan displayed an inferior C4 deposition, when compared to the spores, if incubation was done without chelators (p < 0.001).
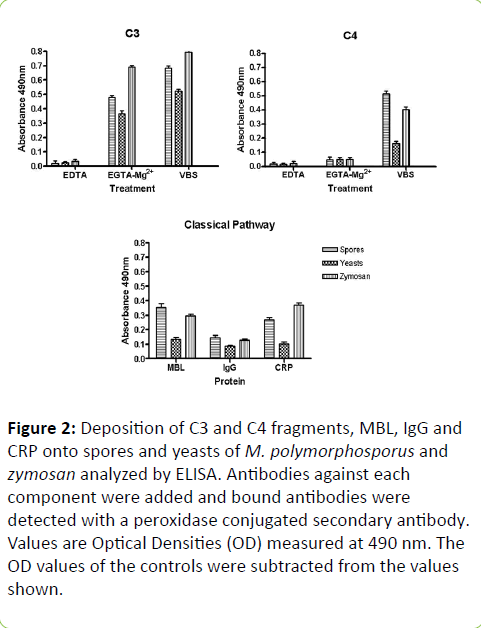
Figure 2: Deposition of C3 and C4 fragments, MBL, IgG and CRP onto spores and yeasts of M. polymorphosporus and zymosan analyzed by ELISA. Antibodies against each component were added and bound antibodies were detected with a peroxidase conjugated secondary antibody. Values are Optical Densities (OD) measured at 490 nm. The OD values of the controls were subtracted from the values shown.
IgG, CRP and MBL detection on fungal surfaces
The incidence of IgG, CRP and MBL on sample surfaces, after activation, was also examined. IgG, CRP and MBL deposition profiles were quite different for both forms tested (Figure 2). The proteins tested were also found on yeasts, however their levels were much lower (p < 0.001). This confirms the presence of C4 fragments, which requires activation by classical or lectin pathways.
MBL and IgG incidence on the positive control, zymosan, were comparable to those examined on the spores (p > 0.05). The results for CRP deposits on zymosan, though, were higher than the spores (p < 0.001).
The incidence of C3 fragments on fungal surfaces
The distribution of C3 fragments deposited onto the fungi was examined with or without EGTA, which chelates Ca2+, essential for classical pathway activation.
Direct fluorescence technique was employed to identify C3 fragments on both M. polymorphosporus spores and yeasts (Figure 3). The tests demonstrated and uniformly fluorescence spread on both forms when incubation occurred in the presence or absence of EGTA-Mg2+. Being as counterstaining with Evan’s blue dye, which represses auto-fluorescence and non-specific antibodies, is accountable for this color spectrum, the presence of EDTA inhibits all pathway activations and thus its results show red fluorescence.
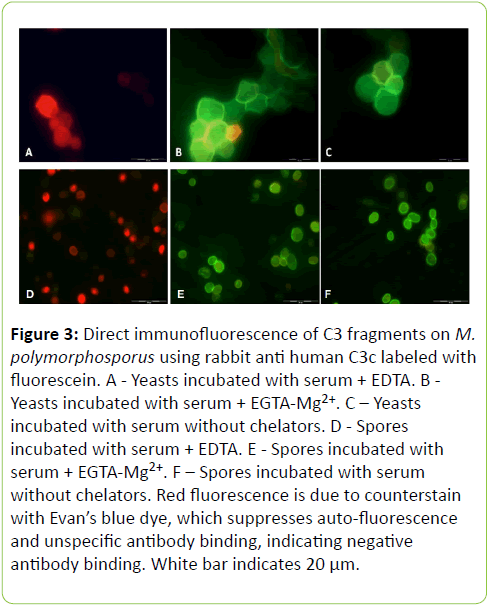
Figure 3: Direct immunofluorescence of C3 fragments on M. polymorphosporus using rabbit anti human C3c labeled with fluorescein. A - Yeasts incubated with serum + EDTA. B - Yeasts incubated with serum + EGTA-Mg2+. C – Yeasts incubated with serum without chelators. D - Spores incubated with serum + EDTA. E - Spores incubated with serum + EGTA-Mg2+. F – Spores incubated with serum without chelators. Red fluorescence is due to counterstain with Evan’s blue dye, which suppresses auto-fluorescence and unspecific antibody binding, indicating negative antibody binding. White bar indicates 20 μm.
While under light microscopy, the relative area of both spore and yeast forms were measured. Fifteen samples of each were used to render the result of 55.2 ± 6.36 μm2 for spores and 621.26 ± 11.64 μm2 for yeasts.
Discussion
The quantity of mucormycosis reports per year increases as times passes and now ranks third in the most frequent invasive mycosis charts [21]. After Rhizopus sp., Mucor species, alongside Absidia sp. and Cunninghamella sp., are mainly responsible for mucormycosis cases [3]. Our previous work has shown that Mucor polymorphosporus mycelia (20 mg/ml) from a clinical specimen could activate the complement system in vitro, presenting close to 40% of complement consumption [22].
Seeing as both forms had full complement consumption when all pathways were liberated or when only the alternative pathway was working, the logical indications is that the alternative pathway in both cases was predominant. The strong complement activation resultant from both spore and yeast forms is corroborated by the intense fluorescence observed after C3 was investigated on their surface. Several fungi, for instance, Aspergillus fumigatus, Blastomyces dermatitidis, Candida albicans and Cryptococcus neoformans have the ability to activate the alternative pathway of the complement system [12,23]. Numerous phylogenetic papers [24-26] state that alternative pathway components are presumably the most primitive complement apparatus, which is in agreement with the thought that countless organisms can access the system by this path.
The cell concentration differences for yeast and spore forms were due to their contrast in size. While spores have an approximate area of 60 μm2, the yeasts are larger with 600 μm2, therefore an adjustment in concentration was necessary. For that reason 108cells/ml was used for spores while 107 were used for yeasts. This similar surface area allowed the ELISA data from both forms to be comparable. C3 deposition onto yeast forms was lower than on spores (p < 0.001), especially when all pathways were allowed. The higher C3 fragment deposition, when all pathways were working together, was probably due to the participation, in some degree, of classical and/or lectin pathways. C3 fragment distribution onto spores and yeast were evenly displayed throughout the entire fungal surfaces, suggesting a competent opsonization of both forms. C3b/iC3b deposited subsequent to complement activation, can be active opsonins, which can be recognized by CR1 [27] and CR3 [28], respectively. Thus, confirmation of deposited C3b is critical to validate an successful complement response with opsonizing fragments, that could likely direct to the phagocytosis of an intruding microbe. Different papers have revealed that C3b and iC3b, that are able to produce ester or amide bonds with microbial surface structures [29], are detected on fungi after activation, such as: Candida albicans [30], Aspergillus fumigatus [31], Cryptococcus neoformans [32], Blastomyces dermatitidis [33], and as a result, could assist binding to phagocytes.
C4 presence was also assayed by ELISA. It was evident that C4 levels on yeasts were much lower than on spores, indicating that there was little C4 participation on yeasts, which normally is necessary for classical and/or lectin pathways. This data was corroborated by the fact that MBL, IgG and CRP levels were lower on yeasts when compared to spores. Since differentiation from spores to hyphae undergoes a yeast-like stage, this also suggest that as M. polymorphosporus shifts to hyphae, complement dependent removal of these cells is diminished. The presence of yeasts of Mucor circinelloides in a patient with bladder infection, although not related to the illness, showed that yeast-like form from this genus is viable in humans [7].
Normally, C3 deposition is a lot quicker by classical than alternative pathway [23], hinting that its usage would be more effective. Alternatively, if the classical pathway is utilized, IgG or IgM are habitually required [34] and, as a rule, adaptive immunity is necessary in order to obtain these antibodies. Since antibody synthesis takes some time, responses towards the antigen would be delayed. Hence, complement activation by the alternative pathway is decisive, as it directly influences antigen presenting to phagocytes, inducing adaptive immunity [27,28]. Recently, we perceived that mycelia from M. polymorphosporus had higher complement consumption when the whole set of pathways were available, contrary to alternative pathway activation [22]. This means that mycelia from M. polymorphosporus, and our tested samples, could employ activation by the classical pathway. On a recent work [6], immunohistological specimens from a patient with mucormycosis revealed the presence of C1q, MBL, IgG and IgM on hyphae of Mucor sp., confirming that Mucor species are able to bind these proteins in vivo. No C3 or C4 fragment deposits were observed on the fungus, indicating inefficient complement activation by hyphae or absence of surface acceptors able to binds these proteins, which is in accordance with our earlier work [22].
Besides permitting activation by the classical pathway, CRP and IgG may also work as opsonins [34,35], being recognized by certain receptors, as FcγR [36,37]. Total complement consumption, could point to anaphylatoxin formation, which would carry out chemoattractant function, attracting neutrophils and dendritic cells [38,39]. Likewise, this could indicate competent microbial elimination.
Given that MBL shows affinity towards mannose rich carbohydrates [40], this would hint that the samples tested may contain the same type of structure. It has been demonstrated mannose residues on Mucor rouxii cell wall composition [41]. Our earlier work probed for MBL on the surface of M. polymorphosporus mycelia and could not identify any occurrence after complement activation [22]. A viable possibility is that there are structural differences between mycelial and spore forms. These differences have been acknowledged in M. rouxii [41].
On a previous study [14] we suggested that spores of an array of Mucor species may have comparable complement activation characteristics. As was reported, all species tested presented the indistinctive complement responses. For the spores of M. polymorphosporus the results were very similar, including C3 and C4 fragments, MBL, CRP and IgG. This indicates that spores from most Mucor species possess a similar profile.
The complement system is a branch of innate immunity, and activation by spores of Mucor sp., which may be present at the beginning of the disease, significantly improves fungal elimination from the patient. As the fungi progresses to hyphae, complement activation becomes impaired, enhancing the chance of developing the infection into mucormycosis, especially in immunocompromised individuals.
Acknowledgments
The present work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Universidade Federal do Rio de Janeiro (UFRJ). A special thanks to Fundação Oswaldo Cruz (FIOCRUZ) for providing sheep’s blood, both Fátima Regina de V. Goulart and Ana Regina M. de Sousa for technical support.
8069
References
- Abdel-Hafez SI(1984) Composition of fungal flora of four cereal grains in Saudi Arabia. Mycopathologia85: 53-57
- Alonso A, Rodriguez SR, Rodriguez SM, Mouchian K, Albonico JFE, et al. (1997)Intersticialpneumonites induced in guinea pigs by the antigens of Rhizopusnigricans. J InvestigAllergolClinImmunol 7: 103-109
- Chayakulkeeree M, Ghannoum MA, Perfect JR (2006) Zygomycosis: the re-emerging fungal infection. Eur J ClinMicrobiol Infect Dis 25: 215-229
- Prabhu RM, Patel R (2004)Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment.ClinMicrobiol Infect S1: 31-47
- Gonzalez CE, Rinaldi MG, Sugar AM (2002)Zygomycosis. Infect Dis Clin North Am 16: 895-914
- Kusaba G, Ohsawa I, Ishii M, Inoshita H, Ohi H, et al. (2010) Evidence of immunopathological traces in mucormycosis: an autopsy case. ClinExpNephrol 14: 396-400
- Cooper BH (1987) A case of pseudoparacoccidioidomycosis: detection of the yeast phase of Mucorcircinelloides in a clinical specimen. Mycopathologia 97: 189-193
- Chan-Tack KM, Nemoy LL, Perencevich EN (2005) Central venous catheter-associated fungemia secondary to mucormycosis.Scand J Infect Dis 37: 925-927
- Chandra S, Woodgyer A (2002) Primary cutaneous zygomycosis due to Mucorcircinelloides. Australas J Dermatol 43: 39-42
- Iwen PC, Sigler L, Noel RK, Freifeld AG (2007) Mucorcircinelloides was identified by molecular methods as a cause of primary cutaneous zygomycosis. J ClinMicrobiol 45: 636-640
- Weitzman I, Della-Latta P, Housey G, Rebatta G (1993) MucorramosissimusSamutsevitsch isolated from a thigh lesion. J ClinMicrobiol 31: 2523-2525
- Speth C, Rambach G, Würzner R, Lass-Flörl C (2008) Complement and fungal pathogens: an update. Mycoses 51: 477-496
- Dunkelberger JR, Song WC (2010) Complement and its role in innate and adaptive immune responses. Cell Res 20: 34-50
- Granja LF, Pinto L, Almeida CA, Alviano DS, Da Silva MH, et al. (2010) Spores of Mucorramosissimus, Mucorplumbeus and Mucorcircinelloides and their ability to activate human complement system in vitro. Med Mycol 48: 278-284
- Marx RS, Forsyth KR, Hentz SK (1982) Mucorales species activation of a serum leukotactic factor. Infect Immun 38: 1217-22
- Lima AO, Silva, WD (1970) Imunologia, Imunopatologia, Alergia-Métodos. Rio de Janeiro: Guanabara Koogan
- Mayer MM (1961) In: Kabat EA, Mayer MM (eds) Experimental Immunochemistry. 2nd ed. Springfield: Thomas Publisher
- Voller A, Bidwell DE (2002) In: Rose NR, Friedman H, Faley J Eds. Manual of Clinical Laboratory Immunology. Washington, American Society for Microbiology 99-109
- Closs O, Aarli JA (1974) Evans blue as counterstain in the demonstration of muscle antibodies by immunofluorescence in myasthenia gravis. J ClinPathol 27: 162-167
- Fine DP (1975) Pneumococcal type-associated variability in alternate complement pathway activation. Infect Immun 12: 772-778
- Bouza E, Muñoz P, Guinea J (2006) Mucormycosis: an emerging disease? ClinMicrobiol Infect 12: 7-23
- Granja LFZ, Pinto L, Alviano DS, Silva MH, Alviano CS, et al. (2008) Activation of Human Complement System by Mucorpolymorphosporus Mycelia. The Open Mycology Journal 2: 94-99
- Kozel TR (1998) Complement activation by pathogenic fungi. Res Immunol 149: 309-320
- Pinto MR, Melillo D, Giacomelli S, Sfyroera G, Lambris JD (2007) Ancient origin of the complement system: emerging invertebrate models. AdvExp Med Biol 598: 372-388
- Zhu Y, Thangamani S, Ho B, Ding JL (2005) The ancient origin of the complement system. EMBO J 24: 382-394
- Smith LC, Clow LA, Terwilliger DP (2001) The ancestral complement system in sea urchins. Immunol Rev 180: 16-34
- Ahearn JM, Fearon DT (1989) Structure and function of the complement receptors CR1 (CD35) and CR2 (CD21). AdvImmunol 46: 183-219
- Rosen H, Law SKA (1990) The leukocyte cell surface receptor(s) for the iC3b product of complement. Curr Top MicrobiolImmunol 153: 99-122
- Law SK, Minich TM, Levine RP (1981) Binding reaction between the third human complement protein and small molecules. Biochemistry 20: 7457-7463
- Kozel TR, Brown RR, Pfrommer GS (1987) Activation and binding of C3 by Candida albicans. Infect Immun 55: 1890-1894
- Kozel TR, Wilson MA, Farrel TP, Levitz SM (1989) Activation of C3 and binding to Aspergillusfumigatus Conidia and Hyphae. Infect Immun 57: 2412-2417
- Young BJ, Kozel TR (1993) Effects of strain variation, serotype, and structural modification on kinetics for activation and binding of C3 to Cryptococcus neoformans. Infect Immun 61: 2966-2972
- Zhang MX, Klein B (1997) Activation, binding, and processing of complement component 3 (C3) by Blastomycesdermatitidis. Infect Immun 65: 1849-1855
- Gadjeva MG, Rouseva MM, Zlatarova AS, Reid KB, Kishore U, et al. (2008) Interaction of Human C1q with IgG and IgM: Revisited. Biochemistry 47: 13093-13102
- Casey R, Newcombe J, McFadden J, Bodman-Smith KB (2008) The acute-phase reactant C-reactive protein binds to phosphorylcholine-expressing Neisseria meningitidis and increases uptake by human phagocytes. Infect Immun 76: 1298-1304
- Sobota A, Strzelecka-Kiliszek A, G?adkowska E, Yoshida K, Mrozi?ska K, et al. (2005) Binding of IgG-opsonized particles to Fc gamma R is an active stage of phagocytosis that involves receptor clustering and phosphorylation. J Immunol 175: 4450-4457
- Mold C, Du Clos TW (2006) C-reactive protein increases cytokine responses to Streptococcus pneumoniae through interactions with Fc gamma receptors. J Immunol 176: 7598-7604
- Markiewski MM, Lambris JD (2007) The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol 171: 715-727
- Gutzmer R, Köther B, Zwirner J, Dijkstra D, Purwar R, et al. (2006) Human plasmacytoid dendritic cells express receptors for anaphylatoxins C3a and C5a and are chemoattracted to C3a and C5a. J Invest Dermatol 126: 2422-2429
- Gadjeva M, Takahashi K, Thiel S (2004) Mannan-binding lectin – a soluble pattern recognition molecule. MolImmunol 41: 113-121
- Bartnicki-Garcia S (1968) Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol 22: 87-108








