Andi Muhammad Ichsan1*, Habibah S. Muhiddin1, Muhammad Affan1, Soraya Arifin1, Budu P1, Soraya Taufik1 and Sudirman Katu2
1Faculty of Medicine, Department of Ophthalmology, Hasanuddin University, Makassar, Indonesia
2Faculty of Medicine, Department of Internal Medicine, Hasanuddin University, Makassar, Indonesia
- *Corresponding Author:
- Andi Muhammad Ichsan
Faculty of Medicine, Department of Ophthalmology
Hasanuddin University, Makassar, Indonesia
Tel: +6281342280880
E-mail: am_ichsan@yahoo.com
Received date: November 13, 2018; Accepted date: November 26, 2018; Published date: November 28, 2018
Citation: Ichsan AM, Muhiddin HS, Affan M, Arfin S, Budu P, et al. (2018) Subretinal Filaria with Macular Hole: A Rare Case. Ann Clin Lab Res Vol. 6 No.4:268. doi:10.21767/2386-5180.100268
Keywords
Subretinal parasite; Filarial worm; Macular hole
Introduction
In many parts of the world, parasitic infections can cause blindness. Some infections are encountered almost exclusively in developing nations, such as infection of nematode and trematode. Poor hygiene, lack of standardized public education, and restricted medical resources associated with a tropical climate; all contribute and promote emergence of specific disease pathogens, vectors, and host reservoirs not found in developed countries [1]. In Indonesia, some areas are still endemic of filariasis. The area where the case was reported 68 cases of filariasis in 2008.
In Indonesia, parasitic infections of the eyes are very rarely reported. A parasitic disease affecting whole part of eye include retinal layers for unilaterally or bilaterally. Onchocercia volvulus, Loa-loa, Brugia malayi and Wucherecia bancrofti have been reported as specific species which can infected retina. The organism can survive up to 4 years in the subretinal space [1].
Case Presentation
A 35-year old man work as a extension of farmer consult in one of district South Sulawesi had sudden blurred vision decreased in the left eye for one-week. Initially it was a central visual field loss then progressively involved the whole visual field, associated with floaters. No history of trauma, particular medicine consumption and inflammation.
The patient neither had any previous history of fever, skin rashes or any treatment for filariasis. The visual acuity was 6/6 in the right eye and 1/60 in the left eye with mild relative afferent pupillary defect. Anterior segment examination in the left eye was normal. Posterior segment examination revealed a white living filarial, approximately three-disc diameters in, moved actively in the macula at the subretinal space (Figures 1 and 2). The worm body was roughly tapered at one end and slightly rounded at the other end, easy to identify with wriggling movements among crisscrossing diffuse subretinal tracks and also found a macular hole. There was no evidence of vasculitis or any retinal haemorrhage.
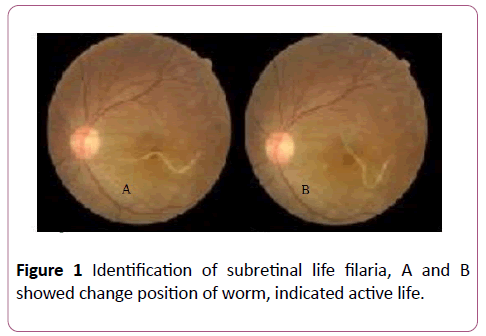
Figure 1: Identification of subretinal life filaria, A and B showed change position of worm, indicated active life.
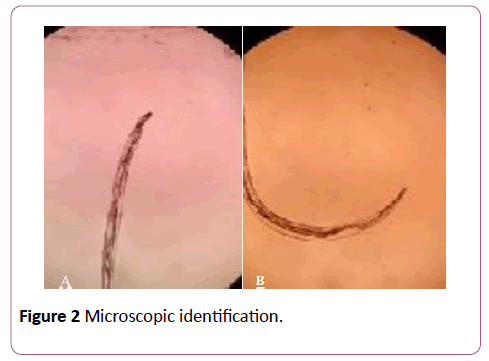
Figure 2: Microscopic identification.
Systemic examination showed no significant finding with no jaundice or hepatosplenomegaly and no cutaneous skin disorder. The blood investigations revealed white blood count was elevated by 15.93 × 103/ul with also elevated eosinophil count (4.2%), normal hemoglobin of 15.7 g/l, normal red blood cell count of 5.39 × 103/ul and normal platelet count of 303 × 103/ul. Urine analysis, Fecal analysis and nocturnal peripheral blood smear for microfilaria were negative. Clinically the diagnose being to intraocular nematode with macular hole.
The patient was consulted to infectious disease specialist and were planned treatment by oral Diethylcarbamazine (DEC) 6 mg/kg body weight three times divided dose a day for 28 weeks and repeated nocturnal peripheral blood smear analysis. DEC would be combined with Albendazole 200 mg in administration of 28-40 weeks. The results of peripheral blood smear analysis are supposed to be positive, but the result was negative so that Doxycycline 100 mg a day for 14 days was given. Evacuation the worm intraocular was planned on following day. Intraoperative, the worm was identified with active motility in premacular area. Peripheral retina seen as grayish-white lesion. The worm was inactivated by laser photocoagulation during pars plana vitrectomy because the motility of worm. Evacuation of a 6 mm worm by iatrogenic retinectomy hole in inferior macula area was performed. Macular hole was identified and treated by Internal Limiting Membrane (ILM) peeling and tamponade gas. Laser photocoagulation were performed around of iatrogenic hole and peripheral retina. Microscopic identification results concluded that the ivory colored object was probably an adult filarial worm of Loa-Loa or Onchocercia volvulus species. Postoperatively, the eye remained quiet, with retained preoperative vision, intact macula and retinal
Discussion
There are many reports of intraocular filariasis. But in Indonesia very rarely, even many areas endemic lymphatic filariasis, such in this case, the patient works as extention of farmer in district area with high filariasis morbidity cases.
The mechanism of the adult worm found in the subretinal area of this patient remains uncertain, but the process of migrant larvae as microfilariae can be slightly explained. Larvae from vector (mosquito or fly) get into the body through the process of cutaneous larva migrants or direct exposure, depend on the spesies [2-7]. In the blood, Larvae can lead to lymphatic tissue, and develop into adult filaria. Adult filaria may excrete microfilariae actively moving in the blood toward the target tissue or feed by the vector. Microfilariae can access intraocular through arteri retina, arteri ciliaris or choroiocapiler, break through the blood retinal barrier and become an adult worm in subretinal [1]. Direct parasitological microscopic examination is necessary for accurate diagnosis, but serological study can also be helpful [3]. In this case, the parasitolgist difficult to identify the filaria species according to Beaver in 1989 reviewed only 6 of 56 cases filariae were removed could be identified [1]. Yamamoto reviewed photocoagulation would make parasitological identification impossible. The worms were evacuated in this case had get laser photocoagulation. But the unsuitable placement of these worms prior to the parasitology examination was the cause of difficulty identified species [3]. This patient performed twice the examination of peripheral blood smear, the result was negative. This may be due to unsuitable retrieval and storage as well as the results of urine and fecal examinations, where samples should be analyzed immediately before two hours. K Myint reported that by the time the worm reaches the subretinal space, systemic markers may not be informative as there is likely to be a time lapse between systemic infestation and intra ocular involvement. Intraocular protection from the worm is interesting and little is known about susceptibity factors to retinal infestation after systemic infection. The Th1/ Th2 immune response may be impaired during infestation and return to normal levels between the recurrent bouts of infection. This may explain why markers of systemic disease such peripheral blood smear analysis, urine and stool shedding are absent, despite persistent survival in the relatively immune privileged subretinal space [3,4]. Serological tests such as IgE and IgM can be performed, serum IgE level will increase by 2-4 weeks and persist for up to 4-6 weeks, while IgM levels will increase 200 days post-infection. But, for this case did not perform the these test [8-10]. This case had many unusual attributes: while the subretinal tracks pointed to trans-retinal migration of the worm with macular damage. Previously reported nematodes were smaller, slow moving, and subretinal [1-5]. The worm did not often report with intraocular inflammation, but there was visual loss, probably because its pre-macular migration and sequestration lead to effects of prolonged subretinal movements. This patient became symptomatic in the usually ‘insidious’ initial stage [3]. However, subclinical trauma to macula due to incessant flagellations by the worm than become macular hole [3]. So in this case the prognosis of visus after therapy may not be promising. Yellow-white or gray-white lesions probably related with damage the outer retina and retinal pigment epithelium by traces of the worm bodies or by inflammation reaction from products of the larva worm such toxic effects, immunologic stimulation excretory or unknown soluble tissue toxins, indicated that this case possible to DUSN condition [2,3,8]. Various types of management for intraocular parasites have been reported. Direct photocoagulation to the worm body has been successfully reported in cases with filaria-like worms and in one case with insect parasites [3]. It has been suggested that photocoagulation denatures the parasite proteins and mitigates the immune reaction [3,5]. If the parasite is located in the posterior pole of the retina, photocoagulation may cause permanent visual impairment, and surgical removal should be selected. Furthermore, photocoagulation would make parasitological identification difficult. Pre-retinal or subretinal parasites were retrieved successfully by pars plana vitrectomy in several reported cases [3,5-10].
This case presented a management dilemma: the pre-foveal parasite could neither be photocoagulated, nor killed by pharmacotherapy, which failed in absence of intraocular inflammation [3,5]. In view of the poor long-term prognosis, we recommend early surgical removal in this case such a case if photocoagulation is not feasible; even in the presence of good vision and no inflammation [7,10]. Endolaser photocoagulations were done to inactivate and evacuated the worm. This case also needs treatment for macular hole by ILM peeling and tamponade gas.
Infectious disease specialist planned oral Diethylcarbamazine (DEC) 6 mg/ kg body weight three times divided dose a day for 28 week to this case [5]. The patient's peripheral blood smear was negative; it was difficult to obtain DEC. So the next treatment option is Albendazole 200 mg, where the patient is difficult to get this medicine, then the choice of therapy given is Doxicyclin 100 mg for 14 days, with the hope this patients can be given Albendazole at the next follow-up.
Conclusion
Adult filarial worms in subretinal space cause severe damage and macular hole by worm motility. However rapid and right management is the primary choice to inhibit damage and to detect the species for systemic therapy. Basically, in particular area of Indonesia is endemic for filaria. Once infect the human body, it may cause various manifestation such as elephantiasis, lymphatic disorders, and so on. In contrast, ocular manifestation especially sub-retinal infection has never been reported nationally. Recently, ministry of health initiate and promote a 5 year (2015-2020) national program to eradicate this parasitic infection. We can suggest that this case may increase awareness that filarial worm can aect human body including eyes that resulting vision threatening or even permanent blindness.
Conflict of Interest
The authors declare no conflict of interests.
23697
References
- Nancy M, Kapil G (2015) Ocular parasitic infections-An overview. Advances in Common Eye Infections 3: 1.
- Freund KB, Sarraf D, Mieler WF, Yannuzzi L (2017) The Retinal Atlas. E-Book. Elsevier p: 480.
- Yamamoto S, Hayashi M, Takeuchi S (1999) Surgically removed submacular nematode. British Journal of Ophthalmology 83: 1088.
- McBurney-Lin S, Khorram D, Gee S, Hoberg EP, Klassen-Fischer MK, et al (2018) A new worm infiltrating the human cornea: A report of three cases. Am J Ophthalmol Case Rep 9: 124-130.
- Farrar J, Hotez PJ, Junghanss T, Kang G, Lalloo D, et al. (2013) Manson's tropical diseases. E-Book. Elsevier Health Sciences.
- Fitzgerald CR, Rubin ML (1974) Intraocular parasite destroyed by photocoagulation. JAMA Ophthalmol 91(2): 162-164.
- Goodart RA, Riekhof FT, Beaver PC (1985) Subretinal nematode. An unusual etiology for uveitis and retinal detachment. Retina (Philadelphia, 5: 87-90.
- Funata M, Custis P, Green WR (1993) Intraocular gnathostomiasis. Retina (Philadelphia, Pa.) 13: 240-244.
- Gupta A, Agarwal A, Dogra MR (1992) Retinal involvement in Wucheria bancrofti filariasis. Acta Ophthalmol 70: 832-835.
- Myint K, Sahay R, Mon S, Saravanan VR, Narendran V, et al. (2006) ÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâ¦ââ¬Ã
ÂWorm in the eyeÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂÃÂ: the rationale for treatment of DUSN in south India. Br J Ophthalmol 90: 1125-1127.








