Key words
|
| |
| Antimicrobial, 4-quinolone, 3, 5-pyrazolidine-dione |
| |
Introduction
|
| |
| The rapid rise in microbial resistance to the traditional antibiotics has necessitated a continuing search for new classes of compounds with novel modes of antimicrobial activity[1]. The 4-quinolone antimicrobials have a number of advantages over other classes of antimicrobial agents. They have wide spectrum of activity, well absorbed orally, have relatively long serum half lives and minimal toxicity[2]. The currently used quinolone derivatives are known to have several drug interactions and adverse side effects[3]. Recently 3, 5- dioxo pyrazolidines were shown to be novel inhibitors of UDP-N-Acetylenolpyruvylglucosamine reductase (MurB) with activity against Gram +Ve bacteria[4]. Similarly a variety of biological activity of substituted 4-Quinolone have been reported and these includes antitubercular[5], antitumor[6], anitifilarial[7], anti- HIV[8] and antimicrobial activity of chloroquinoline containing pyrazoline[9]. Keeping these above facts in view, we considered it for interest to synthesize a few 3,5-pyrazolidinedione substituted 4-quninolone for their antimicrobial activity. |
| |
Experimental
|
| |
|
Chemistry
|
| |
| The chemicals and reagents used in the present project were of AR and LR grade, procured from Aldrich, Hi-Media, Merck, Sigma and Ranbaxy. Melting point of the synthesized compounds were determined by open capillary method and is uncorrected. The purity of compunds was checked by TLC on microplates using silica-gel-G, solvent system was Toluene: Ethanol (95:5) with iodine vapors as detecting agent. Nitrogen estimation was done using elemental analyzer Heraeus carlo Erba 1108, IR spectra of the synthesized compounds were recorded on Shimadzu 8400S FT-IR spectrometer (KBr disc). 1H NMR spectra were recorded on amx- 400 NMR spectrometer (D2O, TMS) and Mass spectra recorded on Jeol Sx 102/DA-600 mass spectrometer/Data System using fast moving bombardment (FAB) technique. |
| |
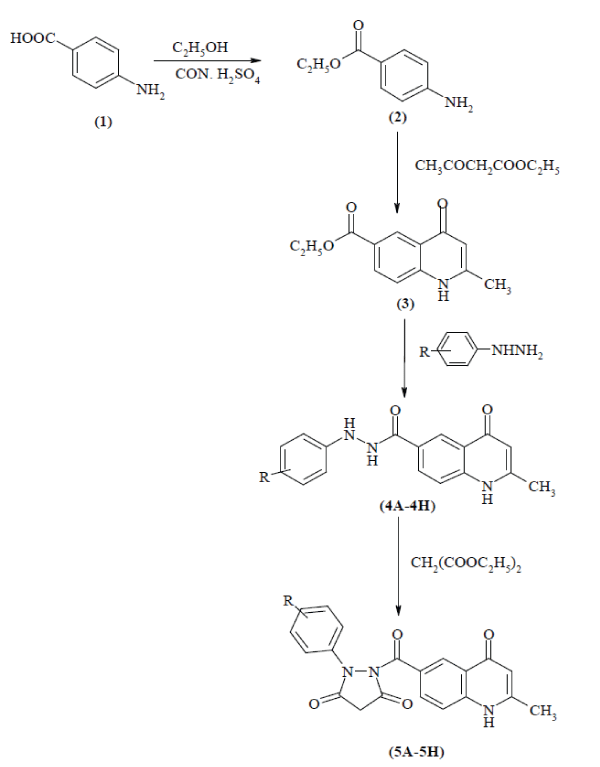
| The title compounds [5A-H] were prepared through several steps as depicted in Fig 1. |
| |
|
Ethyl p-aminobenzoate [2]
|
| |
| Ethyl p-aminobenzoate was prepared by Esterification of PABA by using the reported method[10]. P-amino benzoic acid 0.04 mol (5.48 g) was dissolved in 75mL of ethanol and mixture was heated on a sand bath until the entire solid dissolved. Cooled in room temperature and conc. Sulfuric acid (12.5 mL) was added. A large amount of precipitate formed when sulfuric acid was added, but dissolved during reflux, an air condenser was attached and refluxed for 60-70 min. than mixture was allowed to cool at room temperature and a solution of sodium bicarbonate (10 %) was added to neutralize the excess sulfuric acid. As pH increased, a white precipitate of ethyl p-amino benzoate (benzocaine) was produced. When gas no longer evolved as drop of sodium bicarbonate (pH 8). Prepared product was collected by vacuum filtration. Product was dried in open container and purified from ethanol. Yield: 94.4 %, m.p. 99-101°C. |
| |
|
2-methyl-4-oxo-1,4 dihydro-quinoline-6-carboxylic acid ethyl ester [3]
|
| |
| The compound 2-methyl-4-oxo-1,4 dihydro quinoline-6-carboxylic acid ethyl ester was prepared from Ethyl p-amino benzoate by Conrad-Limpach reaction[11]. A mixture of ethyl p-amino benzoate [2] 0.07 mol (11.55 g), ethyl acetoacetate 0.1 mol (13 g), dioxane (25 mL) and trace of conc. HCl was taken in 250 mL round bottom flask and refluxed at 70-80?C for 3 h, cooled at room temperature and conc. sulfuric acid (20 mL) was added. Than the mixture was refluxed at 190-200?C for 1 h and hot mixture was poured in 500mL of ice/cold water with constant stirring. Separated solid was filtered, dried, and recrystallized from ethanol. Yield: 76.92%, m.p. 265- 267ºC. |
| |
|
2-methyl-4-oxo-1,4 dihydro quinoline-6carboxylic acid N-phenylhydrazides [4A-H]:
|
| |
| A suspension of 2-methyl-4-oxo-1,4 dihydroquinoline- 6-carboxylic acid ethyl ester [3] 0.026mol (6 g) in methanol (10 mL) was prepared. Required phenyl hydrazine 0.05 mol (6 mL) then added to it at room temperature. After stirring, ethanol (20 mL) was added and refluxed for 1 h, cooled at room temperature, filtered and washed the solid with diethyl ether (20 mL) and purified from ethanol[12]. Some common stretching and bending vibrations are as follows IR (KBr) 3282 (N-H), 3039 (Ar, C-H), 2957 (alkanes), 1720 (C=O), 1631,1548,1242 (CONH) 1606 (>C=O, quinolone). The m.p. and % yield of the synthesized compounds were given in Table 1. |
| |
| 1-(2-methyl-4-oxo-1, 4-dihydro quinoline-6- carbonyl)-2-phenyl-pyrazolidine-3, 5-dione [5A-H]: A mixture of 2-methyl-4-oxo-1,4 dihydro quinoline-6carboxylic acid-N’-phenyl hydrazides (0.01mol) [4A-H] were prepared. Diethyl malonate (0.015 mol), ethanol (90mL) and acetic acid (1mL) was added and refluxed for 5hrs. The reaction mixture was left in open dish for 2-3 hrs. The solid precipitate formed was filtered, dried and recrystallized from ethanol[13]. The m.p. and % yield of the synthesized compounds were given in Table 1, Physical data in Table 2 and spectral data of the titled synthesized compounds [5A-H] were given in Table 3. |
| |
|
Antimicrobial evaluation
|
| |
| The synthesized compounds were evaluated against bacterial strains i.e. Bacillus subtilis, Staphylococcus aureus, Escherichia coli and Shigella species and fungal strains i.e. Candida albicans and Aspergillus niger using broth dilution method. The strains were procured from MTCC, Chandigarh, as clinical isolates. Ciprofloxacin and Fluconazole were used as standards for antibacterial and antifungal activity studies respectively. |
| |
|
Antibacterial Testing by Broth Dilution Method
|
| |
| The stock solution was prepared in dimethylsulphoxide (DMSO). The solutions in the test medium furnished the required concentration ranging from 20 to 160 μg/mL. Nutrient broth[14] (beef extract 3 g, peptone 5 g, sodium chloride 5 g and distilled water-q.s. to 1000 mL) was employed as culture media for antibacterial studies. The sterilization of the nutrient broth, culture tubes, pipette and other glassware was done by autoclaving at 15 lb/sq inch pressure for 30 min. Incubation was carried out at 37±1°C for 48 h . The MIC value were recorded as the lowest concentration of the substances that had no visible turbidity using a systronics Turbidometer. All the tests were performed in triplicate for determination of MICs. |
| |
|
Antifungal Testing by Broth Dilution Method
|
| |
| Similarly antifungal activity was carried out. For antifungal studies potato dextrose broth [15] (potato extract 250g, dextrose 20g and distilled water q.s. 1000 mL) was employed. Incubation was carried out at 28±1°C for 72 h. All the tests were performed in triplicate for determination of MICs. |
| |
Results and discussion
|
| |
| A series of novel 1-(2-methyl-4-oxo-1,4- dihydro quinoline-6-carbonyl)-2-(substituted Phenyl)-pyrazolidine-3, 5-diones [5A-5H] were synthesized with different aromatic hydrazides. Para amino benzoic acid (PABA) [1] was converted into Ethyl p-amino benzoate [2] by its esterification. The melting point of PABA is 188-190ºC. The melting point of the ethyl p-amino benzoate is 99-101ºC. The difference in melting point clearly indicates the formation. IR spectrum of the compound [2] clearly shows the vmax at 1311 cm-1 and 1720 cm-1 due to ester (C-O) and ketone (C=O) respectively. Ethyl p-amino benzoate [2] is converted into 2-methyl-4-oxo-1,4 dihydro quinoline-6-carboxylic acid ethyl ester [3] by Conrad-Limpach reaction. The IR spectrum shows carbonyl group (>C=O of 4-quinolone) at 1606 cm-1 and 2º amine (-NH-) at 3284 cm-1 which clearly indicate formation of the compound [3]. |
| |
| Compound 2-methyl-4-oxo-1,4-dihydroquinoline- 6-carboxylic acid-N-Phenyl hydrazide [4A-H] is prepared from [3] with different substituted phenyl hydrazine. Some important peaks of IR spectrum of [4A-H] shows vmax at 1631cm-1, 1548 cm-1 and 1242 cm-1 due to amide (-CONH-) and 1614cm-1 due to (>C=O, quinolone), 1531 cm-1 (N=O asymmetric str), 1332 cm-1 (N=O symmetric str) due to -NO2 group, 757 cm-1 due to (C-Cl) and 2940 cm-1 due to (C-H str of methyl group on benzene ring). In the last step, title compounds [5A-H] was prepared from [4A-H] with diethyl malonate. The IR spectrum of [5A-H] clearly indicates carbonyl group (>C=O of pyrazolidine-3, 5-dione) at 1690cm-1. |
| |
| In 1H-NMR spectra, presence of NMR signals at δ 2.1 ppm (3H, CH3), 3.4 ppm (2H, pyrazolidinedione), 4.67 ppm (1H, NH), 6.86 ppm (1H C3-H), 7.22-7.26 ppm (8H, Ar) clearly indicates the formation. |
| |
| The synthesized compounds were subjected to antimicrobial studies. MICs were determined by the broth dilution method. |
| |
|
Antibacterial activity
|
| |
| The antibacterial screening was done using B. subtilis, S. aureus, E. coli and S. species. Ciprofloxacin was selected as standard drug. Out of all the eight compounds evaluated for antibacterial studies, compound 5G showed appreciable antibacterial activity against all four bacterial strains, (66Tg/mL against S. aureus, 64Tg/mL against B. subtilis, 68Tg/mL against S. species and 66Tg/mL against E. coli). Other compound with some moderate activity was 5A (88Tg/mL against S. aureus, 78Tg/mL against B. subtilis, 82Tg/mL against S. species and 98Tg/mL against E. coli) and 5E (86Tg/mL against S. aureus, 74Tg/mL against B. subtilis , 90Tg/mL against S. species and 88Tg/mL against E. coli). MIC of the tested synthesized compounds are shown in Table 4. |
| |
|
Antifungal activity
|
| |
| Similarly antifungal activity was carried out using fungal strains A. niger and C. albicans. Fluconazole was selected as the standard drug. Out So far as antifungal activity is concerned, the most effective compounds were 5F (62 Tg/mL against C. albicans, 66 Tg/mL against A. niger , 5G (78 Tg/mL against C. albicans, 64 Tg/mL against A. niger.) All compounds have shown moderate antifungal activity against C. albicans and A. niger. Other compounds with moderate antifungal activity were 5A (78 Tg/mL), 5H (82 Tg/mL) and 5C (88 Tg/mL). MIC of the tested synthesized compounds are shown in Table 5. |
| |
| SAR studies of these synthesized compounds also revealed that substitution on benzene ring system which was attached to pyrazolidinedione also affect the activity. Compound with nitro substitution at benzene ring provides more effective compound than alkyl and halogen substitution. Mono substitution is more effective than ‘di’ or ‘tri’ substitution. |
| |
Conclusion
|
| |
| It can be concluded that synthesized fused heterocyclic ring display not only good antifungal activity but also provide good antibacterial activity as well. The effectiveness of the synthesized compounds as antibacterial was found to be greater, when compared to antifungal activity. Compound with nitro substitution on benzene ring is more preferable. These compounds can be considered as lead molecules for future investigations. |
| |
Acknowledgement
|
| |
| Authors are thankful to the Heads, Department of Pharmaceutical Chemistry and Department of Microbiology for kind permission to carry out the work in their departments. The help rendered by IISC, Quest Bangalore for spectral and nitrogen analysis is gratefully acknowledged. |
| |
Tables at a glance
|
 |
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
Table 5 |
|
| |
Figures at a glance
|
 |
| Figure 1 |
|
| |







