Keywords
Curcumin; Anti-inflammatory activity; Spectroscopy; Metal complex
Introduction
Curcumin
The growing public interest in traditional medicine, particularly plant based medicine, has led extensive research on the potential of natural origin substances. Hundreds of studies were conducted to investigate the effect of natural origin compounds on human health and prevention and treatment of chronic diseases. Among studied compounds, polyphenols play an important role in maintenance of health and prevention of diseases. It acts as a strong anti-oxidant and anti-inflammatory agent. Polyphenols also play an important role in regulating immune system and are now being investigated chemopreventive, neuroprotective, cardioprotective, hepatoprotective agents, either acting alone or in combination [1,2]. Polyphenols are derived from many component of the human food including peanuts, dark chocolate, green and black tea, and turmeric. Among polyphenols curcumin is currently one of the most studied one. Curcumin, a typical example of Polyphenol-rich natural remedies, has been used for centuries in Indian traditional medicine (Ayurveda) and Traditional Chinese Medicine (TCM) [2].
Curcumin obtained from the rhizome of turmeric (curcuma longa) a perennial herb belongs to Zingiberaceae family which is cultivated extensively in the extreme south and tropical Asia. The rhizome of this plant is also referred to as the “root” and is the most useful part of the plant for culinary and medicinal purpose. Components of turmeric are named Curcuminoids; it consists of 3-5% Curcuminoids which include mainly curcumin (diferuloyl methane), demethoxycurcumin, and bisdemethoxycurcumin [3].
Turmeric was described as C. longa by Linnaeus and its taxonomic position is as follows:
Class: Liliopsida
Subclass: Commelinids
Order: Zingiberales
Family: Zingiberaceae
Genus: Curcuma
Species: Curcuma longa
The wild turmeric is called C. aromatica and the domestic species is called C. longa (Figures 1 and 2) [4].
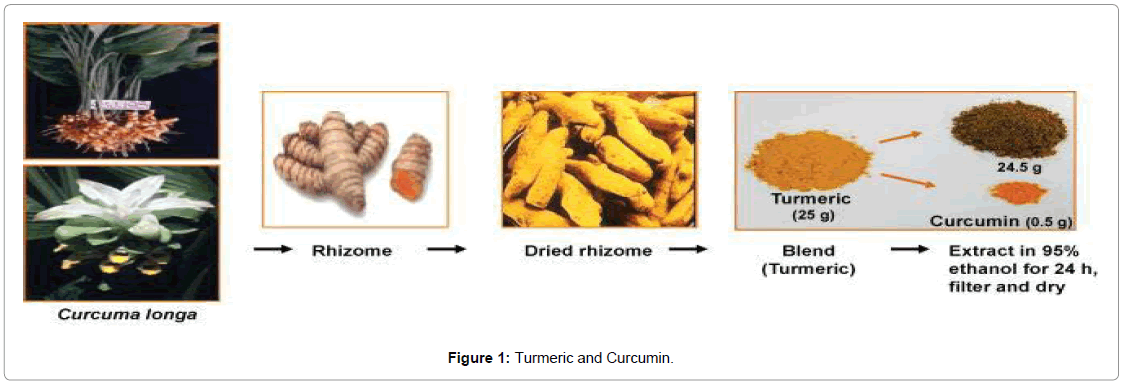
Figure 1: Turmeric and Curcumin.
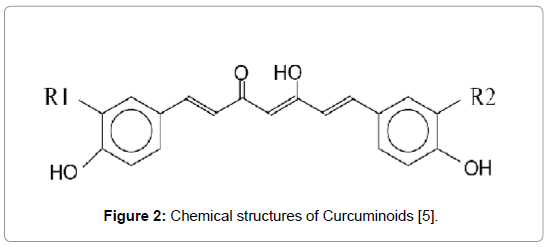
Figure 2: Chemical structures of Curcuminoids [5].
Curcumin: R1 = R2 = OCH3: 1, 7-bis-[4-hydroxy-3 methoxyphenyl]- hepta-1,6- diene-3,5-dione. Chemical formula: C21H20O6; Molecular weight: 368 g/mol. pKa=8.54
Curcumin (diferuloyl methane), is the phytochemical that gives a yellow colour to turmeric and is now recognized as responsible for most of the therapeutic effects [5,6]. It is estimated that 2-5% of turmeric is curcumin. Curcumin was first isolated from turmeric in 1815, and the structure was delineated in 1910 as diferuloyl methane. The structure of Curcumin (C26 H20 06 ) was first described in 1910 by Lampe and Milobedeska (Figure 3).
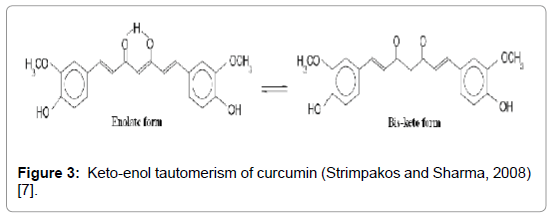
Figure 3: Keto-enol tautomerism of curcumin (Strimpakos and Sharma, 2008) [7].
Curcumin is hydrophobic in nature and frequently soluble in dimethyl sulfoxide (DMSO), acetone, ethanol or oils and insoluble in water [7,8]. It can exist at least in two tautomeric forms, keto and enol [9]. It has mustardy smell and a slightly bitter taste [10,11].
IUPAC defines the transition metals as any element with an incomplete d subshell or that may form stable ions only with an incomplete d subshell [12]. Metals have an esteemed place in medicinal chemistry. Transition metals represent the d block element which includes groups 3 - 12 on the periodic table. Their d shells are in process of filling. This property of transition metals resulted in the foundation of coordination complexes.
A coordination complex or metal complex, is an atom or ion (usually metallic), bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents [13,14]. Many metal-containing compounds consist of coordination complexes [15] .The atom within a ligand that is bonded to the central atom or ion is called the donor atom. A typical complex is bound to several donor atoms, which can be the same or different. Polydentate (multiple bonded) ligands consist of several donor atoms, several of which are bound to the central atom or ion. These complexes are called chelate complexes, the formation of such complexes is called chelation, complexation, and coordination.
The antibacterial study on aqueous extract of C. longa rhizome demonstrated the MIC (minimum inhibitory concentration) value of 4 to 16 g/L and MBC (minimum bactericidal concentration) value of 16 to 32 g/L against S. epidermis ATCC 12228, Staph. aureus ATCC 25923, Klebsiella pneumoniae ATCC 10031, and E. coli ATCC 25922 [16]. The methanol extract of turmeric revealed MIC values of 16 μg/mL and 128 μg/mL against Bacillus subtilis and Staph. aureus, respectively [17]. The study of hexane and ethanol turmeric extract and curcuminoids (from ethyl acetate extract of curcuminoids isolated from C. longa with 86.5% curcumin value) against 24 pathogenic bacteria isolated from the chicken and shrimp showed the highest antimicrobial activity for ethanol extract with the MIC value of 3.91 to 125 ppt [18].
Materials and Methods
Acetone, Chloroform, Methanol, Ethanol, Acetic acid, NaOH (1%) HCl (Dil.), Ferrous Sulphate were required and all the chemicals were purchased from Merck India Pvt. Ltd. Pure curcumin was purchased from New scientific chemical Pvt. Ltd. Dilution has been carried by using distilled water.
Formation of the curcumin metal complex
The curcumin-metal complex was formed using Fe as the central metal atom .The various salts of Fe were taken in the ratio of 1:2 with the curcumin. The complex was synthesized by mixing pure curcumin with salt (Ferric Chloride) at a molar ratio of 1:2 in methanol solution. Curcumin (1.11 g, 3 mmol) was dissolved in 50 ml pure methanol solution and heated at 60°C under a nitrogen atmosphere. Fe salts (6mmol) was dissolved in 100 ml methanol solution and heated for dissolution. The Fe salt methanol solution was added into the curcumin methanol solution, and the Coloured precipitate was produced immediately. The mixture was refluxed for 2 h under nitrogen atmosphere .The solid product was filtered and washed by cold methanol and water to remove the residue reactants, and then the product was dried to get the complex.
Antimicrobial assessment
Pharmacological evaluation involves testing of the microbial susceptibility to chemotherapeutic agents. Determination of antimicrobial effectiveness against specific pathogens is essential for proper therapy. Sensitivity of organisms to antimicrobials may be quantified by the minimum concentration require to inhibit their growth (Minimum inhibitory concentration, MIC). Since it is easier to measure and apply to both bactericidal and bactriostatic drugs, MICs are frequently used. The synthesized compound was tested for their in-vitro growth inhibitory activity against a panel of standard strains of pathogenic microorganism including Gram-positive bacteria, Gram-negative bacteria and the yeast-like pathogenic fungus Candida albicans.
Organism Used
Gram negative bacteria- Escherichia coli (1303)
Gram positive bacteria- P. Aureginosa
Preparation of cultures
Bacteria was cultured in sterile nutrient broth medium which had been autoclaved at 121°C under a pressure of 15 atmospheres for 15 min. and left to grow for 48 h at 37°C in an incubator. The bacterial cultures obtained were diluted with autoclaved Nutrient. This culture served as the inoculums for the antimicrobial experiments.
Preparation of agar plates for Antimicrobial activity
Nutrient agar plates were prepared by mixing Nutrient agar (28 g) in 1000 ml distilled water boiled to dissolve the medium completely. Nutrient agar solution was sterilized by autoclaving at 121ºC for 15 min at 15 lb pressure. After cooling (45ºC), agar solution (25 ml) were poured into sterilized Petri dishes and left to solidify. Agar plates were inoculated with an overnight bacterial culture, using spread plate method after appropriate serial dilutions. Nutrient agar plates were used for E. coli & P. Aureginosa. The curcumin metal complex was aseptically put into the wells (100 μl approx) made in agar plates making lawns of different test cultures viz. E. coli, P. Aureginosa . The Nutrient agar plates were then incubated at 37ºC for 24 h. The diameter of inhibitory zone surrounding disc and antimicrobial curcumin metal complex solution was then measured after 24 hours. Two cross sectional points and the average was taken as the inhibition zone and the size of the zone diameter was measured. The plates were then photographed individually.
Results and Discussion
Spectromertric analysis
UV-visible spectrum analysis: The UV spectra of FeSO4-curcumin complex shows the absorbance value at 428 nm and hence in comparison to the UV spectra of curcumin which shows the absorbance value at 425 nm the shift is hypsochromic shift in nature i.e. towards the lower wavelength (Figures 4-7).
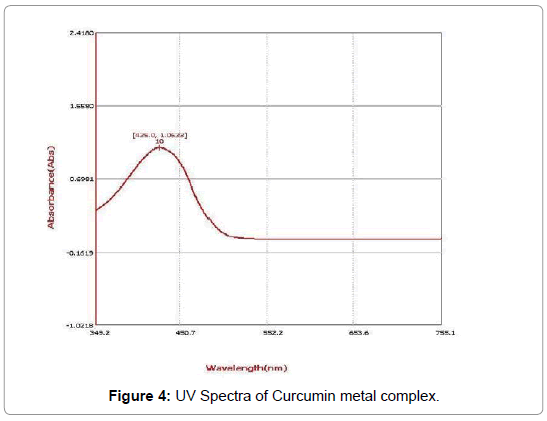
Figure 4: UV Spectra of Curcumin metal complex.
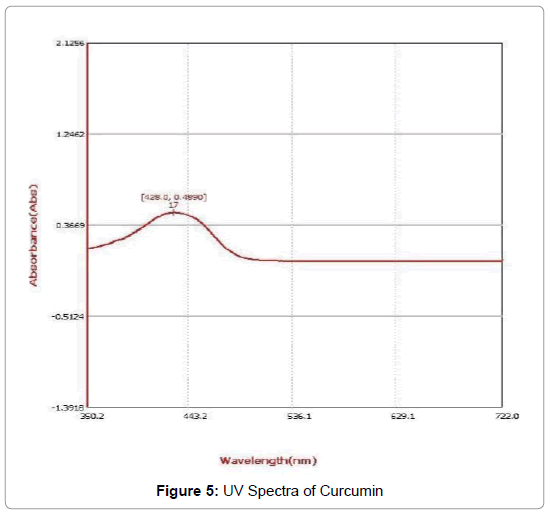
Figure 5: UV Spectra of Curcumin
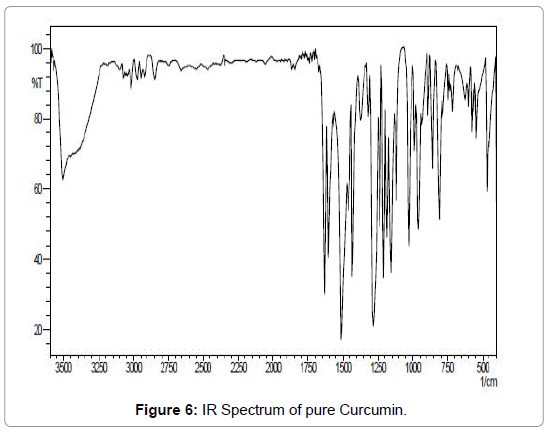
Figure 6: IR Spectrum of pure Curcumin.
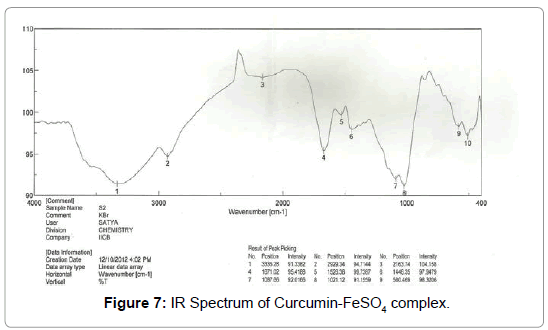
Figure 7: IR Spectrum of Curcumin-FeSO4 complex.
IR spectrum analysis: Important infrared (IR) bands for the Schiff’s base appear at: 3335.28, 2929.34, 2163.74, 1671.02, 1526.38, 1446.35, 1087.66, 1021.12, 580.469 and 511.044 (cm-1).
In the analysis of the compound the hydroxyl group hydrogen bonded (-OH) shows broad band at (3335- 2929) cm-1 with stretching mode of vibration as the aromatic ring show its peak at 2163 cm-1 as it seems to be stretching mode of vibration. The band at 1671 cm-1 in the compound may be assigned to an alkene (C=C) group with strong bonding and stretching vibration. The peak of aromatic ring (C=C) group shows its band at 1526 cm-1 with stretching vibration. Again alkane groups shows its peak at 1446 cm-1 .In the range of (1087-1021) cm-1 with variable bond strength containing two -OH groups present as having asymmetric scissoring and bending vibration. Phenyl ring substitution shows at 576cm-1 and 526 cm-1 respectively as it shows strong bonding property with bending vibration (Figures 8-10) (Table 1).
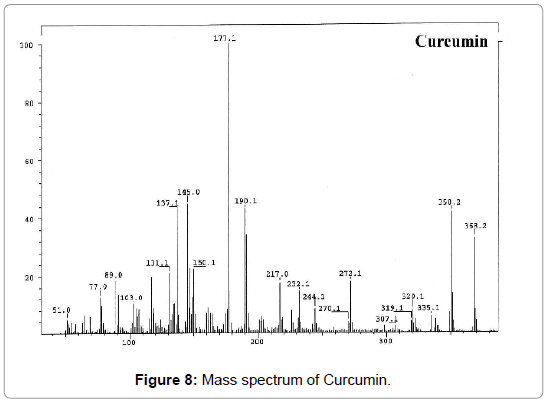
Figure 8: Mass spectrum of Curcumin.
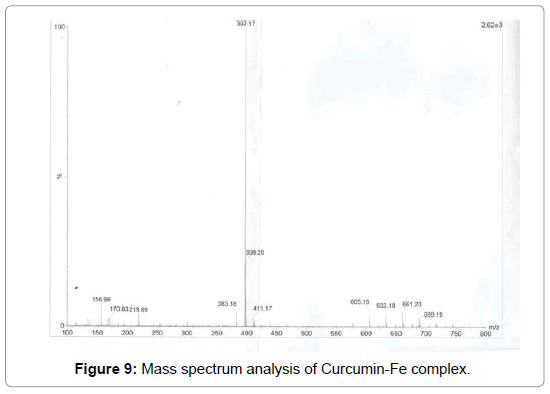
Figure 9: Mass spectrum analysis of Curcumin-Fe complex.
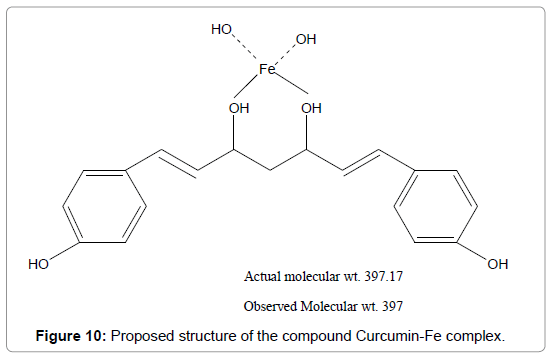
Figure 10: Proposed structure of the compound Curcumin-Fe complex.
| CONCENTRATION |
STANDARD (Curcumin) |
TEST (Curcumin metal complex) |
| Fe sulphate |
| 100µg/ml |
- |
- |
| 200µg/ml |
- |
1.3mm |
| 300µg/ml |
1.21mm |
1.4mm |
| 400µg/ml |
1.41mm |
1.61mm |
Table 1: Zone of inhibition where E.Coli is present.
Mass spectrum analysis: Antibacterial activity: The synthesized curcumin metal complexes have shown more inhibitory activity against bacteria as compared to parental ligands. The highest activity was reported with curcumin Fe complex with 1m growth inhibition for E. coli but not against P. Aureginosa (Figure 11).
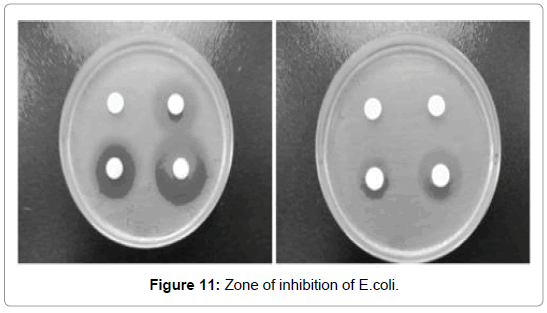
Figure 11: Zone of inhibition of E.coli.
6635
References
- Conney AH (2003) Enzyme induction and dietary chemicals as approaches to cancer chemoprevention: the Seventh DeWitt S. Goodman Lecture. Cancer Res 63: 7005-7031.
- Aggarwal BB, Bhat ID, Ichikawa H, Sethi G, Sandur S, et al. (2006) Biological and Medicinal Properties. 7034: 297.
- Chainani-Wu N (2003) Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa). J Altern Complement Med 9: 161-168.
- Goel A, Kunnumakkara AB, Aggarwal BB (2008) Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol 75: 787-809.
- Ammon HP, Wahl MA (1991) Pharmacology of Curcuma longa. Planta Med 57: 1-7.
- Aggarwal BB1, Sundaram C, Malani N, Ichikawa H (2007) Curcumin: the Indian solid gold. Adv Exp Med Biol 595: 1-75.
- Strimpakos AS, Sharma RA (2008) Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal 10: 511-545.
- Joe B, Vijaykumar M, Lokesh BR (2004) Biological properties of curcumin- cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr 44: 97- 111.
- Payton F, Sandusky P, Alworth WL (2007) NMR study of the solution structure of curcumin. J Nat Prod 70: 143-146.
- Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, et al. (2002) Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev 11: 105-111.
- Chen CM, Fang HC (1997) Chemical analysis of the active principles of Curcuma species. In: Modern Treatise on Chinese Herbal Medicines. Medical Academia 3: 95-105.
- Nic M, Jirat J, Kosata B (2006) Transition element. IUPAC Compendium of Chemical Terminology (Online ed.) 0-9678550-9-8.
- Singh R, Patil SM, Pal G, Ahmad M (2012) Evaluation of in-vivo and in-vitro anti-inflammatory activity of Ajuga bracteosa Wall ex Benth. Asian Pacific Journal of Tropical Disease S404-S407.
- Miriam C, Sosa-Sequera, Suarez O, Dalo NL (2001) Kaurenic acid: An in- vivo experimental study of its anti-inflammatory and antipyretic effects. Source of Support: Research Council (CDCHT) of the Universidad Centroccidental Lisandro Alvarado.
- Wise R, Hart T, Cars O, Streulens M, Helmuth R, et al. (1998) Antimicrobial resistance. Is a major threat to public health. BMJ 317: 609-610.
- Niamsa N, Sittiwet C (2009) Antimicrobial activity of Curcuma longa aqueous extract. Journal of Pharmacology and Toxicology 4: 173-177.
- Ungphaiboon S, Supavita T, Singchangchai P, Sungkarak S, Rattanasuwan P, et al. (2005) Study on antioxidant and antimicrobial activities of turmeric clear liquid soap for wound treatment of HIV patients. Journal of Science and Technology 27: 269-578.

















