Ji-Qun He1#, Lichun Sun2,3,4#*, Jun He3, Chenhong Zhu3, Ping Li3, Jun Lei2, Quanyong He2, Vienna Mackey4, Zhen Lin5, Pengfei Cheng6 and David H Coy4
1The Xiangya Hospital, Central South University, Changsha, China
2The Third Xiangya Hospital, Central South University, Changsha, China
3Sino-US Innovative Bio-Medical Center and Hunan Beautide Pharmaceuticals, Xiangtan, Hunan, China
4Peptide Research Laboratories, Department of Medicine, USA
5Tulane Cancer Center, Tulane University Health Sciences Center, New Orleans, LA70112, USA
6Engineering Research Center for Intelligent Decision Making and Big Data on Industrial Development, Hunan University of Science and Technology, Xiangtan 411201, China
#Equally contributed
*Corresponding Author:
Lichun Sun, Professor of Medicine
Tulane University Health Sciences Center
USA
Tel: 5049881179
E-mail: peptide612@gmail.com; lsun@tulane.edu
Received date: 28 March 2019; Accepted date: 10 April 2019; Published date: 17 April 2019
Citation: He JQ, Sun L, He J, Zhu C, Li P, et al. (2019) The Pathogenesis and Therapeutics of Nasopharyngeal Carcinoma. Health Sci J Vol.13.No.2:642. DOI: 10.36648/1791-809X.1000642
Copyright: © 2019 He JQ, et al. This is an open-access article distributed under the terms of the creative commons attribution license, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Keywords
Nasopharyngeal carcinoma; Epstein–Barr virus; Genetic susceptibility; Viral infection; Environmental factors; Therapeutic
Introduction
Nasopharyngeal carcinoma (NPC) is a type of head and neck carcinoma and arises from nasopharynx epithelium. Compared with other cancers, NPC is rare all over the world, but unique with a geographical and racial distribution. NPC is more prevalent in Southern China, Southeast Asia and Notch Africa (Figure 1) [1,2]. Generally, the incidence rate of NPC is less than 1 case per 100,000 population per year in non-endemic areas. However, the rate in Canton of Southern China is much higher than the global average rate (Figures 2-4) [3-6], even as high as 20-30 per 100 000 per year in certain area of Canton [4]. Surprisingly, the incidence rates are very low in the neighboring Korea and Japan, [2,7]. These indicate that NPC are related to the endemic regions and races. And even NPC was so called as “Cantonese cancer” [8]. As reported, the salted food, smoked meats and other preserved foods that people traditionally take in the endemic regions are believed to have a strong connection with high NPC incidence [2,7]. The modern studies and the accumulated evidences have also identified that NPC initiation and progression is strongly associated with genetic susceptibility and viral infection, besides environmental factors. However, NPC has no obvious symptoms in the early stage, or early symptoms are usually ignored because these early clinical symptoms such as nasal congestion, nasal mass and headache are very similar to cold or flu [7]. Thus, most NPC cases are diagnosed at the late stage, directly resulting in a tough challenge for the effective NPC treatments. Patients with advanced NPC are commonly treated with radiotherapy and chemotherapy due to NPC is radio and chemo-sensitive by comparing with other head and neck carcinomas [7,8]. With scientific advance and technologic progress, novel strategies such as targeted therapy and immunotherapy have been counted on for the future NPC treatments.
Figure 1: The endemic regions of nasopharyngeal carcinoma (NPC) in Asia.
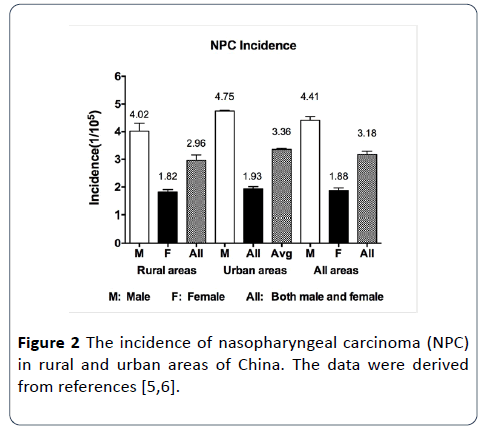
Figure 2: The incidence of nasopharyngeal carcinoma (NPC) in rural and urban areas of China. The data were derived from references [5,6].
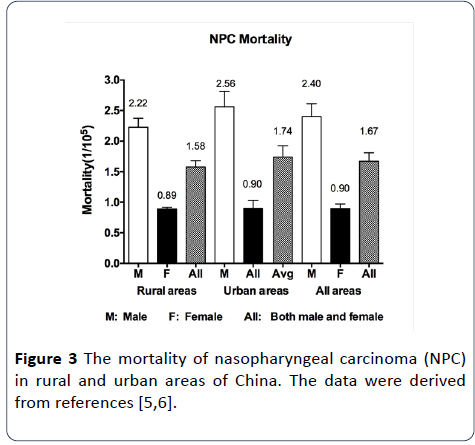
Figure 3: The mortality of nasopharyngeal carcinoma (NPC) in rural and urban areas of China. The data were derived from references [5,6].
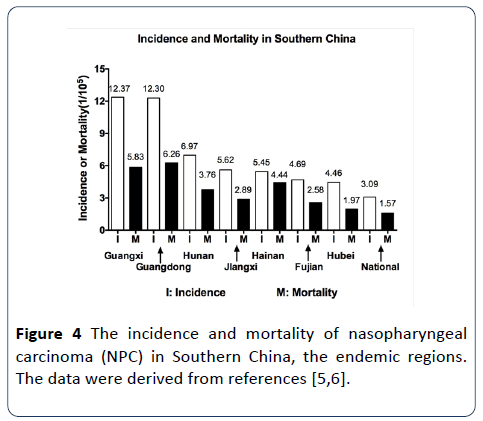
Figure 4: The incidence and mortality of nasopharyngeal carcinoma (NPC) in Southern China, the endemic regions. The data were derived from references [5,6].
The Classification of Nasopharyngeal Carcinoma
Nasopharyngeal carcinoma (NPC) is rare epithelial and squamous cell carcinoma. Based on the difference of NPC cell differentiation, NPC was primarily classified as three subtypes (Type I, II and III) by the World Health Organization (WHO) [1]. The differentiated and keratinizing squamous cell carcinoma was categorized as type I NPC. These NPC cells are well differentiated and can produce keratin. This type of NPC was typically found in the older adult population. Type 2 NPC was categorized to the differentiated and non-keratinizing squamous cell carcinoma, with type III being undifferentiated and non-keratinizing squamous cell carcinoma. More than 95% of NPC cases in Southern China are type II and III or nonkeratinizing carcinomas. Both types II and III NPC are extremely associated with Epstein–Barr virus (EBV), with the infection of EBV being absent in type I NPC [1,2,7]. Up to the difference of NPC growth or metastasis, doctors further used TNM staging system (T: Tumor, N: Node, M: Metastasis) to describe the differences of NPC growth or metastasis. Usually, there are five different stages including stage I, II, III, IVA and IVB [8], or more detailed stages (stage 0, I, II, III, IVA, IVB and cancer recurrence) according to the TNM staging system. In TNM system, each part of TNM was described more details. NPC tumors (T) were staged as T1, T2, T3, and T4 based on tumor size and location. Based on whether NPC spreads to lymph nodes, Nodes (N) were staged as N0, N1, N2 and N3. M0 and M1 stage were assigned for whether NPC metastasizes or not (American Society of Clinical Oncology (ASCO), website: https://www.cancer.net).
The Causes of Nasopharyngeal Carcinoma
It has been documented that NPC development is strongly associated with three key etiological factors, including environmental factors such as chemical carcinogens and toxic pollutants, genetic susceptibility and viral infection, particularly, EBV infection [4,8]. We will discuss more details below.
Environmental factors
Although NPC is rare across all the world, NPC is prevalent in the endemic regions (Canton, Guangxi, Fujian, and Hunan etc.) of Southern China [5,6]. People over there have a long-term traditional custom that they like to eat the salted foods, smoked foods and preserved foods [2,7]. These foods contain the mutagenic chemicals, particularly volatile nitrosamines that can induce carcinogenesis. Canton and Guangxi may be the most prevalent ones of the NPC endemic regions (Figure 4). Scientists did survey and comparison among the Cantonese descendants living in the United States and the Cantonese living in China and the local Americans. They found that the NPC incidence of the Cantonese descendants in the United States is higher than that of the local Americans but lower than that of the Cantonese living in China. Meanwhile, people in the poor suburban areas usually work hard under poor environment and have long-term exposure to toxic pollutants such as dust, paint, smoke and gas. These also increase the NPC risk [3,4]. Other studies reported that the NPC incidence in Hong Kong was decreased due to that the local residents changed their eating habits and reduced their consumption of the traditional foods, particularly the salted fish [4,8]. Furthermore, the NPC incidence in Northern China is much lower than that in Southern China (Figure 4) [2,4-6]. These findings support that environmental factors are strongly related to NPC and play critical roles in initiating NPC development.
Genetic susceptibility
The previous studies have documented the strong association of genetic susceptibility with NPC occurrence. The NPC occurrence and incidence are regional, ethnic and genetic influence. The incidence of NPC in Cantonese descendants living in the United States was higher than that of local Americans. A person with a NPC family history is also NPC susceptible and has a much higher incidence of NPC than an ordinary person. And a person with low immunity is prone to NPC risk. People with EB infection also belong to the susceptible NPC population. Some genomic aberrations and epigenetic alterations that involved in immune response, inflammation, infection and oncogenesis are tightly associated with the increased risk of NPC in the endemic areas. The human leukocyte antigen (HLA) genes locating on chromosome 6p21, with high polymorphisms and many different alleles, encode major histocompatibility complex (MHC) that are cell surface proteins playing the key role of regulating the immune system and triggering body immune response against viral infection. The previous NPC studies among Chinese in Southern China showed that genetic variants in HLAs were associated with NPC susceptibility, resulting in an increase of NPC cases [9,10].
In recent years, more evidences have revealed the associations of genetic alterations with NPC development as the use of various new genetic/epigenetic tools and technologies. The genetic and epigenetic alterations were frequently founded in tumor tissues/cells of NPC patients. In Dr. Fu’s study, the next generation sequencing and analysis showed that the genetic aberrations affected ErbBphosphatidylinositol- 3 kinase signaling in NPC. The allele of cyclooxygenase-2 (COX-2) rs5275 polymorphisms promoted the overexpression of COX-2 in NPC clinical stages in Chinese population, indicating the COX-2 gene variant rs5275 is related to the increased risk of NPC [11]. Inflammation has been demonstrated as a candidate risk factor in NPC tumorigenesis. The interleukin 13 gene (IL-13) that modulates inflammation was found to be susceptible to NPC in the Chinese population. IL-13 rs20541 polymorphisms affected the metastasis of lymph nodes in NPC patients [12]. Genetic analysis disclosed that single nucleotide polymorphisms (SNPs) of GRP78 gene were associated with NPC Chinese patients. The rs3216733 polymorphisms of the GRP78 gene promoter may be NPC susceptible [13]. Another study showed that the monocyte chemoattractant protein-1 (MCP-1) belonging to the cysteinecysteine chemokine family was also related to NPC susceptibility. The rs1024611 polymorphisms of the MCP-1 promoter increased NPC risk [14]. The polymorphisms of both dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin-related (DC-SIGNR) and dendritic cellspecific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN) also act as the risk factors in NPC development [15,16]. DC-SIGN SNPs rs7252229, rs4804803, and rs735240 showed their possibility to increase NPC risk in the Chinese population [16].
Meanwhile, there are many non-coding RNAs (ncRNAs) that are non-coding gene transcripts and are not translated into proteins, including microRNAs, long non-coding RNAs (lncRNAs), small interfering RNAs (siRNAs) and so on. Some of them such as microRNAs and lncRNAs play critical roles in NPC progression and metastasis [17-19]. As reported, microRNA-153 affected NPC tumor cell viability via targeting TGF-β [20] and mircoRNA-629 promoted NPC tumor cell proliferation and migration via targeting PDCD4 [21]. Knockdown of microRNA-342-3p suppressed NPC tumor cell growth and invasion via targeting FOXQ1 [22]. MicroRNA-183 and microRNA-141 are potential risk factors for poor prognosis in NPC patients [23]. LncRNAs are non-coding transcribed RNA molecules with more than 200 nucleotides. A number of studies showed that the dys-regulation of lncRNAs functioned in NPC development [19]. Knockdown of the lncRNA DANCR promoted NPC cell invasion and metastasis via interacting with NF90/NF45 complex and stabilizing HIF-1α [24]. LncRNA LOC284454 promoted NPC invasion via modulating Rho/Rac signaling [25]. Both lncRNA LINC00319 and LncRNA CCAT1 might play an oncogenic role in NPC [26,27]. These new findings may help to design novel therapeutic strategies.
Viral infection
Viral infection, particularly Epstein–Barr virus (EBV) infection, has been demonstrated to be a critical etiological factor in NPC pathogenesis. EBV, the human B-lymphotropic herpesvirus, presents in over 90% of human beings worldwide and normally lasts for whole life without diseased harm. EBV infection are particularly connected with the non-keratinizing types II and III NPC, which account for more than 95% of all NPC cases in Southern China, with the absence of EBV infection in type I NPC cases [3,4]. EBV is also less detected in other head and neck squamous cell carcinomas. Interestingly, EBV is also detected in NPC tumor cells, not in normal nasopharyngeal epithelial cells. Various well-known EB viral oncoproteins including EBV latent membrane proteins (LMP1, LMP2A and LMP2B), EBV nuclear antigens (EBNAs) (EBNA1, EBNA2, 3A, 3B, 3C and -LP), EBV-encoded small RNSs (EBERs) (EBER1 and EBER2), the BamHI A rightward transcripts (BARTs), have been well studied in many NPC studies [1,3,4,17]. Their expressional aberrance in NPC is well connected with NPC development and plays the important roles in NPC progression.
High risky EBV variants characterized by distinct polymorphisms in the EBER locus are reported to have a strong association with NPC [28]. EBV variants with high genetic risk score are found to be much more prevalent in Chinese of Hong Kong than individuals of other geographic regions and also more in NPC than in other EBV-associated cancers. In his study, Verhoeven reported that NF-κB signaling modulated the expression of EBV BamHI-Q-driven EBNA1 in NPC. NF-κB signaling and EBNA1 might maintain a regulatory balance between EBV latent gene expression and NF-κB activity [29]. EBV reactivation changed the balance between EBV and host body and induced NPC pathogenesis. However, EBV-positive NPC is very sensitive to radiotherapy and chemotherapy [8].
EBV is strongly related to NPC in endemic areas as described above. With the application of next generation sequencing and other modern technologies, various EBV noncoding RNAs have been discovered. Some of these EBV RNAs displayed their effects on NPC tumor growth and progression. One study showed that EBV noncoding RNAs from the extracellular vesicles of NPC tumor cells promoted angiogenesis via modulating TLR3/RIG-I-mediated VCAM-1 [30]. Another study proved that EBV-encoded miRNA miR-BART1-5P promoted NPC angiogenesis via regulating AMPK/mTOR/HIF1 signaling in a PTEN-independent manner [31]. EBV-encoded miRNA miRBART13 was upregulated in NPC tissues. This miRNA could promote NPC cell proliferation, tumor growth and metastasis via targeting NKIRAS2/NF-κB signaling [32]. In other studies, the differences in cancer gene copy number alterations (CNAs) between EBV-positive and EBV-negative NPC showed the divergence in oncogenesis. The CNAs occurred more in EBVnegative NPC than in EBV-positive NPC [33]. By analyzing the hypermethylation of tumor-suppressive gene promoter, the hypermethylation of both genes RASSF1A and ESR1 was more frequent in EBV-positive NPC, with that of gene DAPK1 more frequent in EBV-negative NPC. The hypermethylation of RASSF1A might decrease overall survival (OS) rate in EBVpositive NPC, and induce the pathogenesis of EBV-infected NPC [34].
Besides the existence of EBV in majority of NPC cases, particularly type II and III, in the endemic areas, human papillomavirus (HPV) was also observed to be involved in NPC, particularly EBV-absent type I NPC. HPV may act as an etiologic factor in EBV-negative and non-keratinizing NPCs among Caucasians in the non-endemic areas. Ruuskanen et al. investigated the prevalence of EBV and HPV in 150 NPC Caucasian patients in Finnish. They found that 62% of NPC patients were EBV-positive, with 14% being HPV-positive and 24% being negative for both. Their OS rates were higher in EBV-/HPV-positive patients than in EBV/HPV-negative patients. Both EBV and HPV may be the important prognostic factors for NPC in non-endemic areas [35]. Maxwell et al also demonstrated that HPV may be the etiologic factor in certain EBV-negative and non-keratinizing NPC patients in North Americans [36]. Meanwhile, Huang and his team analyzed the incidence of HPV-associated NPC in Southern China, the NPC endemic area. In total 1328 patients, 92% were EBV-positive, 7.7% were HPV-positive, with only 0.6% being co-infected with both EBV and HPV. They found that the prevalence and coincidence of EBV and HPV in NPC patients was extremely low. However, the EBV-negative and HPV-positive NPC patients showed better local tumor control and survival after radiotherapy [37]. These studies suggest that HPV infection is involved in NPC progression. Moreover, the infection of EBV and HPV seems like they are exclusive [7,35,36].
Therapeutic Treatments
NPC is prevalent in certain areas worldwide, with the involvement of multiple factors including genetic susceptibility, EBV infection and environmental exposure to chemical carcinogens. Patients at early stage of NPC have no obvious symptoms, with the most recognized symptoms being neck swelling, nasopharyngeal mass, nasal congestion, headache, cranial nerve palsy etc. These symptoms may be misjudged as flu or most likely ignored. Thus, patients are at late stage of NPC once they have been diagnosed. Besides surgery, patients are usually treated with radiotherapy and chemotherapy due to NPC is highly radio-sensitive and chemo-sensitive [8]. Certain novel therapies such as EBV-based immunotherapy, epigenetic therapy, EBV-based gene therapy and targeted therapy, have also been applied for the NPC treatments [4,8]. In his study, Zhang reported that ATR (ATM (ataxia telangiectasia mutation) and Rad-3 related) activated by EBV facilitated resistance to cisplatin or 5-fluorouracil in NPC chemotherapy [38]. ATR knockdown may effectively treat EBVpositive and chemotherapy-resistant NPC [38].
Currently, immunotherapy such as the drugs associated with PD-L1 (programmed death ligand 1) and PD-1 (PD-L1 receptor) are popular and well known across the world. In their study, Liu et al. found that the low expression of PD-L1 acted as an independent adverse prognostic factor for disease-free survival (DFS) while the high expression of PD-L1 acted as an independent prognostic factor for OS. Their findings supported a strong correlation of the low PD-L1 expression with local recurrence in EBV-positive NPC after radiotherapy [39]. In another study, EBV-encoded miRNA was found to increase radiotherapy resistance in NPC treatments. Upregulatiion of EBV miRNA or miR-BART4 promoted NPC tumor cell proliferation and NPC progression and consequently result in radiotherapy resistance while downregulation of miR-BART4 inhibited cell proliferation and metastasis and further facilitated NPC radiotherapy sensitivity [40]. NPC is radiosensitive, but has a high frequency of failures in NPC treatments due to NPC metastasis. Thus, the failures in NPC treatments frequently result in NPC recurrence. In another study, both CDX2 (Caudal type homeobox 2) and NOX4 (NADPH oxidase 4) were found to be associated with NPC recurrence [41]. The expression of CDX2 and NOX4 was detected in recurrent NPC tumor tissues, but not in other NPC tumor tissues. Thus, targeting these proteins or blocking the associated signaling networks may be a potential and novel therapeutics for the treatments of patients with NPC recurrence [41]. Fibronectin is an extracellular matrix glycoprotein and plays a key role in cell growth, cell migration and cell differentiation. Fibronectin 1 promoted NPC cell migration and invasion via increasing the expression of MMP2 and MMP9. Moreover, the high expression of fibronectin 1 suppressed cell apoptosis in NPC via increasing the expression of BCL2 and the nuclear localization of P65. Thus, Fibronectin 1 may be a potential target for new anti-NPC drug development [42]. EBV reactivation and genomic instability are associated with NPC progression. EBV immediate-early protein BRLF1 is a transcriptional activator. BRLF1 was found to induce the switch from latency to lytic viral replication in the EBV infection cells, resulting in genomic instability and malignancy in NPC. Therefore, targeting BRLF1 may be a new and potential drug strategy [43].
Exosomes are naturally membrane-derived nanovesicles with their range of 30-100 nm in diameter. Exosomes are released by many types of cells and secreted into the extracellular environments [44]. Exosomes can carry proteins, DNA, microRNAs, other small molecule compounds. Particularly, tumor cell-derived exosomes contain tumorspecific antigens and tumor-associated biomarkers. Moreover, exosomes are well tolerated, with lower toxicity and less immunogenicity. These provided potential opportunities for the development of exosome-based therapeutics [44,45]. Particularly, in EBV-positive NPC, EBV product-containing exosomes may be used as diagnostic markers and therapeutic targets for EBV-positive NPC [44,45]. These exosomes have been widely used as drug delivery vehicles in cancer treatment, implicating a possible use in NPC therapeutics.
Conclusion
NPC is different from other head and neck carcinomas, with majority of NPC cases being in the limited endemic areas, particularly in Southern China, and being infected with EBV. Although NPC is radio-sensitive and chemo-sensitive, NPC recurrence after radiotherapy and chemotherapy frequently occurs with the failures of treatments. It is urgent need to develop novel therapeutic drugs or technologies. EBV plays critical roles in NPV development. It is important and necessary to unravel EBV and EBV-associated signaling network. This will help to promote the traditional treatments or develop novel therapeutics. For instance, EBV infection is strongly associated with NPC while this virus primarily targeting B-lymphocytes and epithelial cells [46]. It is promising to develop EBV-targeting immunotherapy. Meanwhile, a focus in these new fields as exosomes, noncoding RNAs, receptor-targeted drugs, could be considered for the potential drug development.
Acknowledgement
This work was supported by Xiangtan Institute of Industrial Technology Collaborative Innovation, Xiangtan Science and Technology Bureau, Natural Science Foundation of Hunan Province (2007JJ60522019JJ40479), the Natural Science Foundation of Hunan Province (2007JJ6052), Zhishan Plan Program of the Third Xiangya Hospital, Central South University ((2017)15), The Human Philosophy Social Science Fund Project (18YBA150).
24357
References
- Chou J, Lin YC, Kim J, You L, Xu Z, et al. (2008) Nasopharyngeal carcinoma--review of the molecular mechanisms of tumorigenesis. Head Neck 30: 946-963.
- Yu MC, Yuan JM (2002) Epidemiology of nasopharyngeal carcinoma. Cancer Biology 12: 421-429.
- Paul P, Deka H, Malakar AK, Halder B, Chakraborty S (2018) Nasopharyngeal carcinoma: understanding its molecular biology at a fine scale. Eur J Cancer Prev 27: 33-41.
- Tao Q, Chan AT (2007) Nasopharyngeal carcinoma: molecular pathogenesis and therapeutic developments. Expert Rev Mol Med 9: 1-24.
- Wei KR, Zheng RS, Zhang SW, Liang ZH, Li ZM, et al. (2017) Incidence and mortality of Nasopharyngeal carcinoma in China, 2013. Chin J Cancer 36: 90.
- Zhang S, Zeng H, Sun K, Chen W, He J, et al. (2018) Incidence and mortality of Nasopharyngeal carcinoma in China, 2014. China J Oncol 40: 566-571.
- Chua MLK, Wee JTS, Hui EP, Chan ATC (2016) Nasopharyngeal carcinoma. Lancet 387: 1012-1024.
- Ho, C.S (2017) Beating 'Guangdong cancer': a review and update on nasopharyngeal cancer. Hong Kong Med J 23: 497-502.
- Wang TM, Zhou T, He YQ, Xue WQ, Zhang JB, et al. (2018) Fine-mapping of HLA class I and class II genes identified two independent novel variants associated with nasopharyngeal carcinoma susceptibility. Cancer Med 7: 6308-6316.
- Lu CC, Chen JC, Jin YT, Yang HB, Chan SH, et al. (2003) Genetic susceptibility to nasopharyngeal carcinoma within the HLA-A locus in Taiwanese. Int J Cancer 103: 745-751.
- Fu J, Li Z, Li N (2018) The association between COX-2 gene rs5275 polymorphism and Nasopharyngeal carcinoma risk. Pathol Res Pract 214: 1579-1582.
- Wang R, Qin HM, Liao BY, Yang FL, Wang JL (2018) Genetic polymorphisms in interleukin 13 gene with the susceptibility to nasopharyngeal carcinoma in a Chinese population. Cytokine 115: 121-126.
- Wang R, Qin HM, Qin L, Wei JX, Wei YX, et al. (2018) Genetic association of promoter in GRP78 gene with nasopharyngeal carcinoma in a Chinese population. Int J Clin Oncol 24: 359-365.
- Niu Y, Zhou G, Wang Y, Qin J, Ping J, et al. (2018) Association of MCP-1 promoter polymorphism with susceptibility to nasopharyngeal carcinoma. J Cell Biochem 120: 6661-6670.
- Ning S, Yao M, Wu Y, Zhou X, Zhong C, et al. (2018) Correlation of variable repeat number in the neck regions of DC-SIGN and DC-SIGNR with susceptibility to nasopharyngeal carcinoma in a Chinese population. Cancer Manag Res 10: 3193-3198.
- Li S, Lu Z, Yao M, Ning S, Wu Y, et al. (2017) Association of single-nucleotide polymorphisms in dc-sign with nasopharyngeal carcinoma susceptibility. Dis Markers 2017: 6309754.
- Zhao CX, Zhu W, Ba ZQ, Xu HJ, Liu WD, et al. (2018) The regulatory network of nasopharyngeal carcinoma metastasis with a focus on EBV, lncRNAs and miRNAs. Am J Cancer Res 8: 2185-2209.
- Nie Y, Liu X, Qu S, Song E, Zou H, et al. (2013) Long non-coding RNA HOTAIR is an independent prognostic marker for nasopharyngeal carcinoma progression and survival. Cancer Sci 104: 458-464.
- He R, Hu Z, Wang Q, Luo W, Li J, et al. (2017) The role of long non-coding RNAs in nasopharyngeal carcinoma: As systemic review. Oncotarget 8: 16075-16083.
- Guo G, Zhang Y, Hu L, Bian X (2019) MicroRNA-153 affects nasopharyngeal cancer cell viability by targeting TGF-beta2. Oncol Lett 17: 646-651.
- Zheng YQ, Bai YF, Yang S, Cui YR, Wang YP, et al. (2019) MircoRNA-629 promotes proliferation, invasion and migration of nasopharyngeal carcinoma through targeting PDCD4. Eur Rev Med Pharmacol Sci 23: 207-216.
- Cui Z, Zhao Y (2019) MicroRNA-342-3p targets FOXQ1 to suppress the aggressive phenotype of nasopharyngeal carcinoma cells. BMC Cancer 19: 104.
- Lian J, Li Y, Yu M (2019) MicroRNA-183 and microRNA-141 are potential risk factors for poor prognosis in patients with nasopharyngeal carcinoma. Oncol Lett 17: 1172-1176.
- Wen X, Liu X, Mao YP, Yang XJ, Wang YQ et al. (2018) Long non-coding RNA DANCR stabilizes HIF-1alpha and promotes metastasis by interacting with NF90/NF45 complex in nasopharyngeal carcinoma. Theranostics 8: 5676-5689.
- Fan C, Wang J, Tang Y, Wang Y, Xiong F, et al. (2018) Long non-coding RNA LOC284454 promotes migration and invasion of nasopharyngeal carcinoma via modulating the Rho/Rac signaling pathway. Carcinogenesis [Epub ahead of print].
- Song P, Yin SC (2019) Long non-coding RNA 319 facilitates nasopharyngeal carcinoma carcinogenesis through regulation of miR-1207-5p/KLF12 axis. Gene 680: 51-58.
- Dong Y, Yuan H, Jin G (2018) Identification of long non-coding RNA CCAT1 as an oncogene in nasopharyngeal carcinoma. Oncol Lett 16: 2750-2756.
- Hui KF, Chan TF, Yang W, Shen JJ, Lam KP, et al. (2018) High risk Epstein-Barr virus variants characterized by distinct polymorphisms in the EBER locus are strongly associated with nasopharyngeal carcinoma. Int J Cancer [Epub ahead of print].
- Verhoeven RJA, Tong S, Zong J, Chen Y, Tsao SW, et al. (2018) NF-kappaB Signaling Regulates Epstein-Barr Virus BamHI-Q-Driven EBNA1 Expression. Cancers (Basel) 10: 4.
- Cheng S, Li Z, He J, Fu S, Duan Y, et al. (2019) Epstein-Barr virus noncoding RNAs from the extracellular vesicles of nasopharyngeal carcinoma (NPC) cells promote angiogenesis via TLR3/RIG-I-mediated VCAM-1 expression. Biochim Biophys Acta Mol Basis Dis [Epub ahead of print].
- Lyu X, Wang J, Guo X, Wu G, Jiao Y, et al. (2018) EBV-miR-BART1-5P activates AMPK/mTOR/HIF1 pathway via a PTEN independent manner to promote glycolysis and angiogenesis in nasopharyngeal carcinoma. PLoS Pathog 14: e1007484.
- Xu YJ, Zhou R, Zong JF, Lin WS, Tong S, et al. (2019) Epstein-Barr virus-coded miR-BART13 promotes nasopharyngeal carcinoma cell growth and metastasis via targeting of the NKIRAS2/NF-kappaB pathway. Cancer Lett 447: 33-40.
- Ooft ML, van Ipenburg J, van de Loo RJM, de Jong R, Moelans CB, et al. (2018) Differences in cancer gene copy number alterations between Epstein-Barr virus-positive and Epstein-Barr virus-negative nasopharyngeal carcinoma. Head Neck 40: 1986-1998.
- Ooft ML, van Ipenburg J, van Loo R, de Jong R, Moelans C, et al. (2018) Molecular profile of nasopharyngeal carcinoma: analysing tumour suppressor gene promoter hypermethylation by multiplex ligation-dependent probe amplification. J Clin Pathol 71: 351-359.
- Ruuskanen M, Irjala H, Minn H, Vahlberg T, Randen-Brady R, et al. (2019) Epstein-Barr virus and human papillomaviruses as favorable prognostic factors in nasopharyngeal carcinoma: A nationwide study in Finland. Head Neck 41: 349-357.
- Maxwell JH, Kumar B, Feng FY, McHugh JB, Cordell KG, et al. (2010) HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck 32: 562-567.
- Huang WB, Chan JYW, Liu DL (2018) Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: Multicenter study from an endemic area in Southern China. Cancer 124: 530-536.
- Zhang B, Cui B, Du J, Shen X, Wang K, et al. (2019) ATR activated by EB virus facilitates chemotherapy resistance to cisplatin or 5-fluorouracil in human nasopharyngeal carcinoma. Cancer Manag Res 11: 573-585.
- Liu YJ, Tsang NM, Hsueh C, Yeh CJ, Ueng SH, et al. (2018) Low PD-L1 expression strongly correlates with local recurrence in epstein-barr virus-positive nasopharyngeal carcinoma after radiation-based therapy. Cancers (Basel) 10: 10.
- Wu Q, Han T, Sheng X, Zhang N, Wang P, et al. (2018) Downregulation of EB virus miR-BART4 inhibits proliferation and aggressiveness while promoting radiosensitivity of nasopharyngeal carcinoma. Biomed Pharmacother 108: 741-751.
- Chen G, Hao H, Ai JW (2018) Regulatory role of CDX2 and NOX4 expression associated with recurrent nasopharyngeal carcinoma. Eur Rev Med Pharmacol Sci 22: 450-455.
- Wang J, Deng L, Huang J, Cai R, Zhu X, et al. (2017) High expression of Fibronectin suppresses apoptosis through the NF-kappaB pathway and is associated with migration in nasopharyngeal carcinoma. Am J Transl Res 9: 4502-4511.
- Huang SY, Wu CC, Cheng YJ, Chou SP, Jiang YJ, et al. (2017) Epstein-Barr virus BRLF1 induces genomic instability and progressive malignancy in nasopharyngeal carcinoma cells. Oncotarget 8: 78948-78964.
- Sun L, He Q, Qin Z, Lei J, Feng B (2017) Exosome and its applications as a novel drug delivery system. Clin Oncology 2: 1-3.
- Teow SY, Liew K, Khoo AS, Peh SC (2017) Pathogenic role of exosomes in Epstein-Barr Virus (EBV)-associated cancers. Int J Biol Sci 13: 1276-1286.
- Teow SY, Yap HY, Peh SC (2017) Epstein-Barr Virus as a promising immunotherapeutic target for nasopharyngeal carcinoma treatment. J Pathog 2017: 7349268.









