Keywords
Gastric carcinoma; AP-3; Drug-targeting; Cell proliferation; Cell apoptosis; Tumor growth
Introduction
Gastric carcinoma is a refractory disease that seriously threatens human health worldwide. According to the GLOBOCAN estimates of cancer incidence and mortality produced by the International Agency for Research on Cancer, the global incidence and mortality of gastric cancer are 5.6% and 7.7% respectively in 2020 [1]. Genetic and environmental factors are the main factors of gastric carcinoma [2]. Genetic factors include GOLPH3 (Golgi Phosphoprotein 3) and Akt/mTOR pathway, TLR2 (toll like receptor-2), MTA2 (Metastasis Associated 1 Family Member 2) and Coronins. The main environmental factors are Helicobacter pylori (H. pylori) infection and unhealthy diet. The proliferation of H. pylori leads to a series of complex inflammation and immune responses, which eventually develops into gastric carcinoma. Long-term high intake of salted fish, sour pickled cabbage, processed meat, saturated fat, refined grains and total carbohydrates and other unhealthy eating habits can also induce gastric carcinoma.
The current therapeutic targets for gastric carcinoma are VEGFR, TKI, EGFR, HER2, PI3K-AKT-MTOR pathway, HGF-C-MET pathway, CD9 and Galectin-7. Most of the drugs available or under investigation are small molecules that usually displayed potent anti-cancer efficacy and serious side effects. Thus, targeted cancer therapy is a hot topic in the fields of anti-cancer drug development. One of the strategies is to couple the efficacious cytotoxic agents to specific drug delivery vehicles such as monoclonal antibodies, short peptides and certain unique proteins [3-6]. The new drug conjugates could deliver non-specific drugs to the targeted sites via the specific delivery vehicles so as to enhance the anti-cancer efficacy and reduce side effects. In our previous studies, we also demonstrated that various conjugates could extremely increase the anti-tumor activities of the cytotoxic agents while reducing the administered doses and side effects [7-9].
Presently, we selected and tested certain cytotoxic agents such as ansamitocin P-3 (AP-3), colchicine and paclitaxel in gastric cancercells in order to select one of them for further development of drug conjugates potentially applied to treat patients carrying gastric carcinoma. Via serial experiments, we found that AP-3 was extremely potent and might be used to develop a receptor-specific drug conjugate against gastric carcinoma.
Materials and Methods
Materials
The compounds ansamitocin P-3 (AP-3), colchicine and paclitaxel were all purchased from MCE (Med Chem Express, NJ, USA), with their purity over 98%. The primary antibodies b-actin(#ab8227), p21(#ab109520), p63(#ab124762), PCNA (#ab92552) and Bcl- 2(#ab32124) and secondary antibodies(Alexa Fluor 680, # ab175773) were from Abcam (Cambridge, MA, USA).
Cell culture
The gastric cancer SGC-7901 cells purchased from BNCC (Beijing Nightingale Consultation of Culture, China) were cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS; Gibcoby life technologies) and incubated in a humidified incubator (Thermo Fisher Scientific, Waltham, MA, USA) at 37°C with 5% CO2.
Cell viability assay
Cell viability assay was determined by following the manufacturer’s protocol of Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan). Briefly, cells were seeded in 96-well cell culture plates at a density of 8×103 cells/well, with different concentrations of compounds added to each well, respectively. The contents in wells were mixed and continuously incubated at 37°C for 72 hours (h). Then 10 μL of CCK-8 solution was added and the plates were incubated for another 1-4 h. The OD values were measured at 450 nm by a microplate reader (Bio Tek, USA).
Cell cycle analysis
Cells (2×105 cells /well) were seeded into 6-well plates and incubated with different concentrations of AP-3 (0, 0.2, 0.4 and 0.8 nM), colchicine (0, 10, 20 and 40 nM) and paclitaxel (0, 3, 6 and 12 nM) for 24 h, respectively. The treated cells were digested with 0.25% trypsin (non EDTA) and harvested. The cell suspension was centrifuged at 1500 rpm for 5 minutes (min). The cells were harvested, washed twice with PBS, fixed with 70% cold ethanol at -20°C for 24h, centrifuged at 4000 rpm for 2 minutes. With the supernatants removed, cells were suspended in 500 μL of PBS with 0.25% Triton-X 100, and incubated on ice for 15 min. After centrifuged, the supernatants were discarded, each tube was added with 500 μL PBS containing 10 μg/mL RNase A and 20 μg/mL PI, and incubated in the dark place at room temperature for 30 min. Finally, the cell samples were placed in Falcon tubes and detected by a CytoFLEX flow cytometry.
Cell apoptosis analysis
Cells were seeded into 6-well plates with 2×105 cells/well, and treated respectively with AP-3, colchicine and paclitaxel at different concentrations at 37°C for 48 h. Then cell apoptosis assay was carried out according to the manufacturer’s instructions. Briefly, the treated cells were collected by centrifugation at 1500 rpm for 5 min. After washed twice with PBS, cells were suspended in 100 μL binding buffer and were incubated with 5 μL FITC-AV and 5 μL PI for 15 min in dark at room temperature. Subsequently, additional 400 μL binding buffer was added to each and analysis was performed by a CytoFLEX flow cytometer.
Quantitative polymerase chain reaction (qPCR) assay
SGC-7901 cells were plated into 6-well plates with the cell density of 2×105 cells/well, and incubated with the tested compounds at different concentrations for 8 h and 24 h, respectively. Total RNAs were extracted using HP total RNA Kit (OMEGA, USA) and was reverse transcribed into complementary DNAs (cDNAs). Following cDNA synthesis, qPCR reactions were performed in triplicate for each of the individual samples using the SYBR Green (Genecopoeia, USA) detection method by a StepOne PCR machine (Bio-Rad). The primer sequences are shown in Table 1. RNA expression was normalized to that of b-actin purchased from Genecopoeia. The relative RNA expression was calculated through the 2−ΔΔCt method.
| Primer |
|
5’---3’ |
| PCNA |
F |
TTAGCTCCAGCGGTGTAAAC |
| R |
TTTGGACATACTGGTGAGGTTC |
| p63 |
F |
CGTGTTATTGATGCTGTGCG |
| R |
GAAGTCATTCCACTCATCTCGG |
| p21 |
F |
TGTCACTGTCTTGTACCCTTG |
| R |
GCGTTTGGAGTGGTAGAAATC |
| Bcl-2 |
F |
TTGTGGCCTTCTTTGAGTTCGGTG |
| R |
GGTGCCGGTTCAGGTACTCAGTCA |
Table 1 The sequences of primers designed for qPCR analysis of genes PCNA, p63, p21 and Bcl-2.
Western blot assay
SGC-7901 cells (2×105/well) were seeded into 6-cell plates and treated with AP-3, colchicine and paclitaxel for 24 h and 48 h, respectively. Then the treated cells were lysed in RIPA Lysis Buffer (Promoter Biotechnology, China) supplemented with protease inhibitor cocktail (MCE, USA). Proteins from each sample were loaded into wells of 12.5% SDS-PAGE gels, separated by the electrophoresis, and transferred to NC membranes. Then the membranes were subsequently blocked by TBST buffer containing 5% (w/v) skimmed milk for 30 min, incubated with each of the primary antibodies (b-actin, p21, p63, PCNA and Bcl- 2), and secondary antibodies of goat-anti rabbit, respectively. After incubation, the NC membranes were washed four times for 5 min each and eventually visualized by ODYSSEY CLx Imager (Near-Infrared Western Blot Detection).
Xenograft mouse model
Female BALB / c nude mice aged 4-6 week-old were purchased and reared in SPF animal house for one week to adapt to the new environment. SGC-7901 cells (5 × 106 cells in 200 μL) were inoculated into the right flank of each mouse by subcutaneous injection. After tumors grown up with the tumor sizes reaching 100-300 mm3 (Tumor volume = 0.5 × Length × Width2), tumor-carrying mice were given by tail vein injection with 200 μL of the tested compound AP-3 at the dose of 0.5 mg/kg (first time) or 0.1 mg/kg (last three times) in the experimental group (n=6), with 200 μL normal saline containing 10% alcohol used in the control group (n=6). Mice were treated once a week for four weeks. Tumor volumes and body weights were measured every other day since the first administration.
Statistical analysis
The experimental data are presented as mean ± SD values from at least three independent experiments. Statistical differences between groups were analyzed by Student’s t-test using the software Graphpad Prism 6.0, with the symbols *, ** and *** represent significant difference of p<0.05, p<0.01 and p<0.001, respectively.
Results
The suppressive effects of the tested compounds on cell proliferation
The three small molecule compounds were tested for their cytotoxic efficacy against the growth of gastric cancer SGC-7901 cells. As shown in Figure 1, all of them displayed potent inhibitory activities, with the IC50 values being 0.61 ± 0.16 nM for AP-3, 14.73±1.73 nM for colchicine and 6.51 ± 1.02 nM for paclitaxel, respectively. Particularly, AP-3 is the strongest one and has ten times more potent than the others. The similar results also have been observed in other gastric cancer cells (data not shown). These findings suggested that AP-3 might be a potential anti-tumor drug to be used for drug conjugates via coupled with delivery vehicles such as peptides or monoclonal antibodies (Table 1).
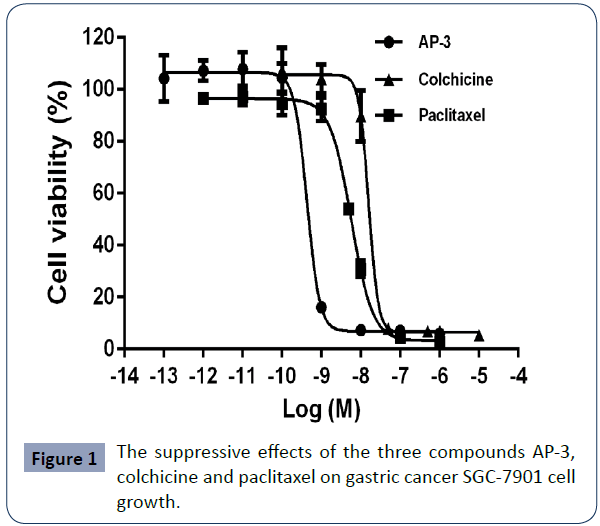
Figure 1 The suppressive effects of the three compounds AP-3, colchicine and paclitaxel on gastric cancer SGC-7901 cell growth.
The effects of the tested compounds on cell cycle progression
Via flow cytometry analysis, we further investigated the effects of these compounds on cell cycle progression. As shown in Figure 2 and Table 2, these compounds induced cell cycle arrest at G2/M phase and in a dose-dependent manner. Compared to the control, AP-3 induced an increase at G2/M phase from 14.29 % ± 5.24% (control) to 60.52 ± 5.93%, 81.95 ± 8.15% and 79.76 ± 13.14% at the concentrations of 0.2, 0.4 and 0.8 nM, respectively, with colchicine-induced increase being 17.34 ± 3.18% (10 nM), 72.94 ± 9.08% (20 nM) and 78.11 ± 10.21% (40 nM), and paclitaxel-induced increase being 30.46 ± 3.03% (3 nM), 49.46 ± 10.78% (6 nM) and 84.42 ± 6.17% (12 nM). The results (Table 2 and Figure 2) suggested that SGC-7901 cells were arrested by AP-3, colchicine and paclitaxel at the G2/M phase. In particular, AP-3 displayed more potency than the other two.
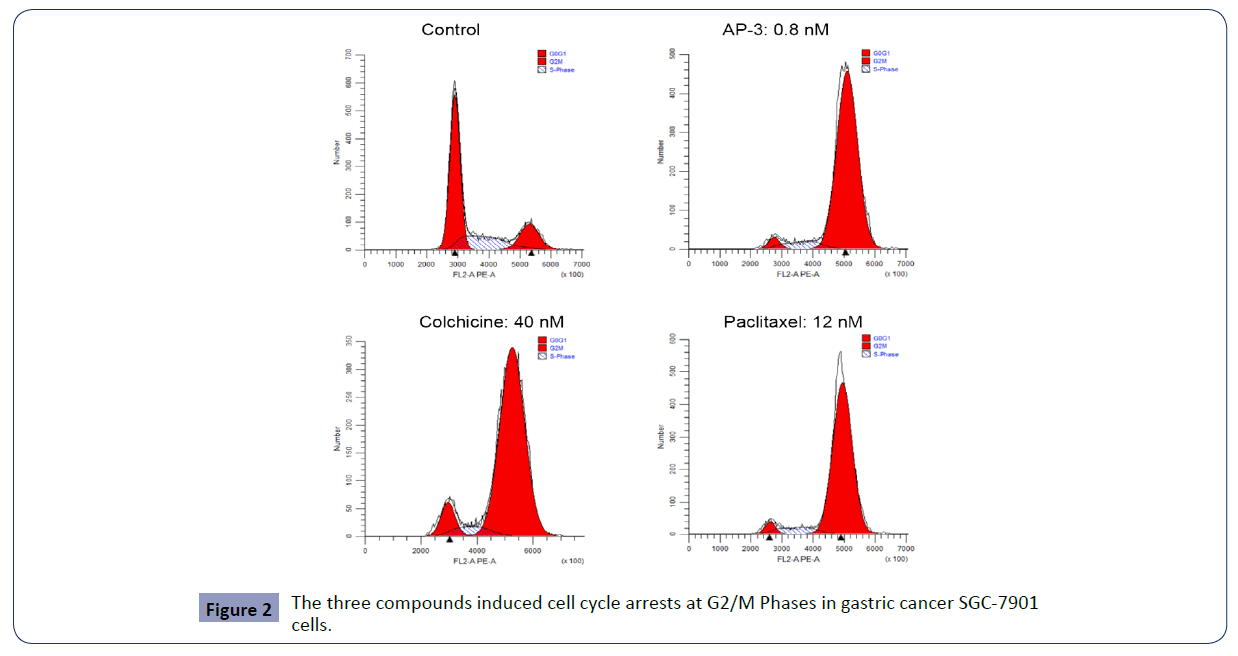
Figure 2 The three compounds induced cell cycle arrests at G2/M Phases in gastric cancer SGC-7901 cells.
| Compounds |
Concentration (nM) |
Phases (%) |
| G0/G1 |
S |
G2/M |
| Control |
0 |
64.80 ± 6.76 |
20.91 ± 1.53 |
14.29 ± 5.24 |
| AP-3 |
0.2 |
25.67 ± 11.58 |
13.81 ± 5.66 |
60.52 ± 5.93 |
| 0.4 |
8.13 ± 4.09 |
9.93 ± 4.07 |
81.95 ± 8.15 |
| 0.8 |
8.45 ± 7.42 |
6.81 ± 2.43 |
79.76 ± 13.14 |
| Colchicine |
10 |
61.53 ± 8.24 |
21.14 ± 5.06 |
17.34 ± 3.18 |
| 20 |
16.89 ± 10.32 |
10.17 ± 1.25 |
72.94 ± 9.08 |
| 40 |
16.25 ± 10.86 |
5.64 ± 0.65 |
78.11 ± 10.21 |
| Paclitaxel |
3 |
40.47 ± 5.45 |
29.08 ± 2.42 |
30.46 ± 3.03 |
| 6 |
16.85 ± 0.51 |
33.20 ± 11.29 |
49.96 ± 10.78 |
| 12 |
5.78 ± 2.79 |
9.81 ± 3.37 |
84.42 ± 6.17 |
Table 2 The tested compounds induced cell cycle arrests at G2/M phases and in a dose-dependent manner.
The effects of the tested compounds on cell apoptosis
We also investigated whether these compounds affected cell apoptosis using Annexin V-FITC/PI double staining kit, and found all three compounds at different concentrations induced either early apoptosis or late apoptosis in SGC-7901 cells compared to the control. According to Table 3 and Figure 3, AP-3 at the concentrations of 0, 0.2, 0.4 and 0.8 nM raised the total apoptosis rates from 4.42 ± 4.95% (control) to 13.89 ±4.62 % (0.2 nM), 19.33 ± 3.15% (0.4 nM) and 23.43 ± 2.45% (0.8 nM), respectively. And colchicine at the concentration of 10, 20 and 40 nM increased the apoptosis rates to 10.19 ± 3.93% (10 nM), 23.60 ± 1.27% (20 nM)and 26.83 ± 6.78% (40 nM), with the apoptosis induced by paclitaxel at the concentration of 3, 6 and 12 nM increased to 11.10 ± 1.17% (3 nM), 12.83 ± 0.62% (6 nM) and 28.24 ± 6.85% (12 nM), respectively (Table 3). Our results suggested that the three AP-3, colchicine and paclitaxel could induce SGC-7901 cell apoptosis in a dose-dependent manner (Figure 4).
| Compounds |
Concentration (nM) |
Apoptosis (%) |
| Early stage |
Late stage |
Total |
| |
0 |
0.37 ± 0.52 |
4.05 ± 4.43 |
4.42 ± 4.95 |
| AP-3 |
0.2 |
3.46 ± 1.41 |
10.44 ± 6.03 |
13.89 ± 4.62 |
| 0.4 |
6.67 ± 2.81 |
12.66 ± 4.16 |
19.33 ± 1.35 |
| 0.8 |
8.38 ± 2.29 |
15.05 ± 4.74 |
23.43 ± 2.45 |
| Colchicine |
10 |
2.45 ± 0.18 |
7.75 ± 3.76 |
10.19 ± 3.93 |
| 20 |
10.60 ± 0.71 |
13.00 ± 0.57 |
23.60 ± 1.27 |
| 40 |
13.28 ± 6.15 |
13.55 ± 0.64 |
26.83 ± 6.78 |
| Paclitaxel |
3 |
3.78 ± 1.58 |
7.32 ± 0.41 |
11.10 ± 1.17 |
| 6 |
6.07 ± 0.83 |
6.76 ± 0.21 |
12.83 ± 0.62 |
| 12 |
11.84 ± 6.74 |
16.4 ± 3.11 |
28.24 ± 6.85 |
Table 3 The tested compounds induced gastric cancer SGC-7901 cell apoptosis in a dose-dependent manner.
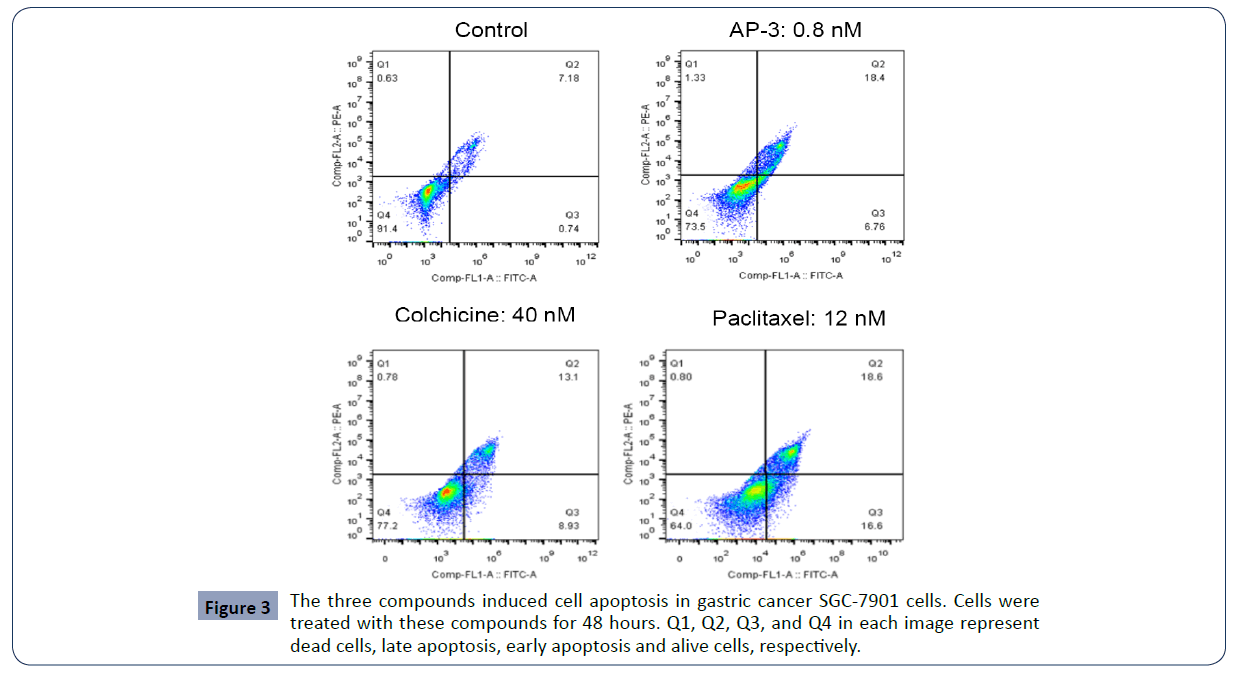
Figure 3 The three compounds induced cell apoptosis in gastric cancer SGC-7901 cells. Cells were treated with these compounds for 48 hours. Q1, Q2, Q3, and Q4 in each image represent dead cells, late apoptosis, early apoptosis and alive cells, respectively.
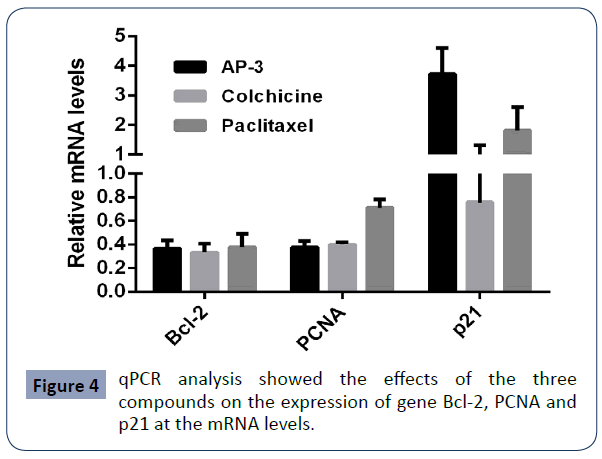
Figure 4 qPCR analysis showed the effects of the three compounds on the expression of gene Bcl-2, PCNA and p21 at the mRNA levels.
The effects of the tested compounds on the expression of the associated genes
Based on the assays described above, we found all these tested compounds could effectively induced cell growth suppression, cell cycle arrest and cell apoptosis. We further investigated the expression of such associated genes as PCNA (a cell proliferation marker), Bcl-2 (an apoptosis marker), p21 (cell cycle regulator and a tumor suppressor) and p63 (a transcription factor and a tumor marker) at the transcriptional level and the translational level. We firstly did qPCR assays and found that all three compounds decreased the expression of Bcl-2 and PCNA at the mRNA level, while both AP-3 and paclitaxel increased the expression of p21, with no significant change under the treatment of colchicine. We also observed that p63 was undetectable. We further did western blot assay and found a slight decrease of PCNA in SGC- 7901 cells treated with all these compounds. Meanwhile, a high decrease of Bcl-2 was induced with the treatments of colchicine and paclitaxel, but a slight decrease treated with AP-3. The increase of p21 was clearly enhanced with the treatment of AP-3 and paclitaxel, with no significant change under the treatment of colchicine, identical to that observed from qPCR assay (Figure 5).
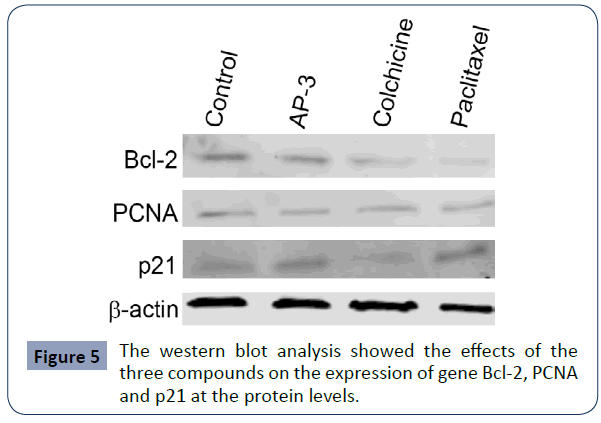
Figure 5 The western blot analysis showed the effects of the three compounds on the expression of gene Bcl-2, PCNA and p21 at the protein levels.
The suppressive effects of the compound AP-3 on tumor growth
The findings from the serial in vitro assays above supported that the said compound AP-3 was the most potent one among the tested ones and might be a potential molecule for the development of drug-targeting conjugates. Thus, in order to know how it works in in vivo assay, we further investigated its anti-tumor efficacy in the xenograft mouse models. SGC-7901 tumors were grown in nude mice and further treated with AP- 3. AP-3 was given to each mouse by tail vein injection once a week for total four weeks. As shown in Figure 6A, at day 12, the average tumor volumes in the control group increased from 248.51 ± 31.29 mm3 to 2412.18 ± 552.94 mm3, with in increased rate of 870.66%. However, the tumor volumes in the tested group decreased from 155.92 ± 18.93 mm3 to 52.72 ± 27.41 mm3, with a decreased rate of -66.18%, showing tumor shrinking and a maximal inhibitory rate of 104.76% in compared to the control. However, with the given doses decreased and over, tumor growth gradually rebounded back. At the last day (day 30), the tumor volumes increased to 1270.84 ± 210.11 mm3. Meanwhile, we observed severe side effects from AP-3 treatment. In AP-3 group, all mice were more inactive and lost their bodyweight (Figure 6B). Especially, one mouse died at day 3 after given with high dose of 0.5 mg/kg, predicting AP-3 itself might have a small drug window and not be druggable.
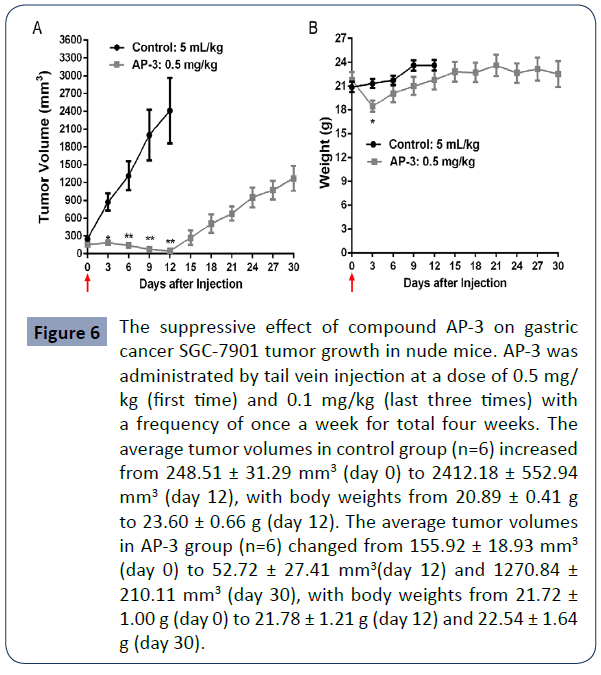
Figure 6 The suppressive effect of compound AP-3 on gastric cancer SGC-7901 tumor growth in nude mice. AP-3 was administrated by tail vein injection at a dose of 0.5 mg/ kg (first time) and 0.1 mg/kg (last three times) with a frequency of once a week for total four weeks. The average tumor volumes in control group (n=6) increased from 248.51 ± 31.29 mm3 (day 0) to 2412.18 ± 552.94 mm3 (day 12), with body weights from 20.89 ± 0.41 g to 23.60 ± 0.66 g (day 12). The average tumor volumes in AP-3 group (n=6) changed from 155.92 ± 18.93 mm3 (day 0) to 52.72 ± 27.41 mmy(day 12) and 1270.84 ± 210.11 mmy (day 30), with body weights from 21.72 ± 1.00 g (day 0) to 21.78 ± 1.21 g (day 12) and 22.54 ± 1.64 g (day 30).
Discussion
Due to traditional chemotherapy usually resulting in serious side effects, scientists gradually put more of their interests on drug-targeting strategies. Antibodies, peptides and proteins are generally used as drug delivery vehicles. In our previous studies, we also identified that peptides displayed their efficacious functions to deliver small molecules or oligo DNA to the target sites through ligand-receptor interactions and quick internalization. In our present study, we attempt to find an ideal small molecule compound and further synthesize a drug conjugate via coupling this compound to a peptide or protein vehicle.
Ansamitocin P-3 (AP-3), a microtubule inhibitor, is a potent maytansinoid anti-tumor agent [10-20]. Colchicine is a neutral lipophilic alkaloid with strong anti-tumor activity and weak anti-inflammatory activity [21,22]. Its mechanism of anti-tumor is similar to AP-3 through the inhibition of microtubule formation [23,24]. Paclitaxel is a natural anti-tumor drug that stabilizes the polymerization of tubulin. It is currently used to treat patients with many cancers such as ovarian cancer, breast carcinoma and lung cancer due to its broad-spectrum anticancer activity[25-28]. In our present study, these classic anti-tumor compounds were chosen to pre-test for their anti-tumor efficacy in gastric cancer cells, all three displayed potent activities in the suppression of cell proliferation and the induction of cell apoptosis. AP-3 was even more potent than other two. The gene expressional analysis at the protein level with western blot assay showed that the compound-induced expressional changes of the associated genes Bcl-2, PCNA and p21 were similar to that at mRNA level with qPCR assay. The in vivo assay further demonstrated that AP-3 could significantly suppress the growth of gastric tumors in nude mice, but resulting in severe side effects with a heavy loss of bodyweight and inactive behavior at a dose of 0.5 mg/ kg. On the other hand, AP-3 likely showed no or less anti-tumor activity at the low dose of 0.1 mg/kg. Thus, AP-3 itself might not be druggable although it is super potent. The strategy of drug-targeting conjugates might provide a potential opportunity for the clinical application of AP-3. Our next step is to further couple AP-3 to a drug delivery vehicle. We expect this new AP-3 conjugate could enhance anti-tumor activity of AP-3 and reduce its side effects.
To summarize, drug-targeting strategies are currently hot topic in the field of drug research and development. Reportedly, many new drug conjugates have been successfully developed via coupling various cytotoxic chemical molecules to delivery vehicles. Our preliminary data also supported that the made drug conjugates had more potent anti-tumor efficacy than the pure molecules and extremely reduced the side effects. These findings predict these new conjugates may have a great opportunity in the near future.
Acknowledgements
We would greatly acknowledge the supports from Shenzhen Science and Technology Program (Grant No: KQTD20170810154011370), Xiangtan Institute of Industrial Technology Collaborative Innovation, and Xiangtan Science and Technology.
36548
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin.
- Majeed W, Iftikhar A, Khaliq T, Aslam B, Muzaffar H, et al. (2016) Gastric Carcinoma: Recent Trends in Diagnostic Biomarkers and Molecular Targeted Therapies. Asian Pac J Cancer Prev 17:3053-3060.
- Chau CH, Steeg PS, Figg WD (2019) Antibody-drug conjugates for cancer. Lancet 394:793-804.
- Wang S, Liu S,Zhang Y, He J, Coy DH, et al. (2020) Human Serum Albumin (HSA) and Its Applications as a Drug Delivery Vehicle. Health Sci J 14: 2.
- White BH, Whalen K, Kriksciukaite K, Alargova R, Yeung TA, et al. (2019) Discovery of an SSTR2-Targeting Maytansinoid Conjugate (PEN-221) with Potent Activity in Vitro and in Vivo. J Med Chem 62:2708-2719.
- Trail PA, Dubowchik GM, Lowinger TB (2018) Antibody drug conjugates for treatment of breast cancer: Novel targets and diverse approaches in ADC design. Pharmacol Ther 181:126-142.
- Moody TW, Sun LC, Mantey SA, Pradhan T, Mackey LV, et al. (2006) In vitro and in vivo antitumor effects of cytotoxic camptothecin-bombesin conjugates are mediated by specific interaction with cellular bombesin receptors. J Pharmacol Exp Ther 318:1265-1272.
- Sun LC, Luo J, Mackey LV, Fuselier JA, Coy DH (2007) A conjugate of camptothecin and a somatostatinanalog against prostate cancer cell invasion via a possible signaling pathway involving PI3K/Akt, alphaVbeta3/alphaVbeta5 and MMP-2/-9. Cancer Lett 246:157-166.
- Sun LC, Luo J, Mackey VL, Fuselier JA, Coy DH (2007) Effects of camptothecin on tumor cell proliferation and angiogenesis when coupled to a bombesinanalog used as a targeted delivery vector. Anticancer Drugs 18:341-348.
- Wang X, Wang R, Kang Q, Bai L (2020) The Antitumor Agent Ansamitocin P-3 Binds to Cell Division Protein FtsZ in Actinosynnemapretiosum. Biomolecules 10.
- Du ZQ, Zhang Y, Qian ZG, Xiao H, Zhong JJ (2017) Combination of traditional mutation and metabolic engineering to enhance ansamitocin P-3 production in Actinosynnemapretiosum. Biotechnol Bioeng 114:2794-806.
- Du ZQ, Zhang Y, Qian ZG, Xiao H, Zhong JJ (2018) Erratum for "Combination of traditional mutation and metabolic engineering to enhance ansamitocin P-3 production in Actinosynnemapretiosum" (Vol. 114, Issue 12, pp. 2794-2806). Biotechnol Bioeng 115:2668.
- Du ZQ, Zhong JJ (2018) Rational approach to improve ansamitocin P-3 production by integrating pathway engineering and substrate feeding in Actinosynnemapretiosum. Biotechnol Bioeng 115:2456-2466.
- Fan Y, Gao Y, Zhou J, Wei L, Chen J, et al. (2014) Process optimization with alternative carbon sources and modulation of secondary metabolism for enhanced ansamitocin P-3 production in Actinosynnemapretiosum. J Biotechnol 192 Pt A:1-10.
- Gao Y, Fan Y, Nambou K, Wei L, Liu Z, et al. (2014) Enhancement of ansamitocin P-3 production in Actinosynnemapretiosum by a synergistic effect of glycerol and glucose. J IndMicrobiolBiotechnol 41:143-152.
- Izawa M, Wada Y, Kasahara F, Asai M, Kishi T (1981) Hydroxylation of ansamitocin P-3. J Antibiot 34:1591-1595.
- Jia Y, Zhong JJ (2011) Enhanced production of ansamitocin P-3 by addition of Mg2+ in fermentation of Actinosynnemapretiosum. Bioresour Technol 102:10147-10150.
- Li J, Sun R, Ning X, Wang X, Wang Z (2018) Genome-Scale Metabolic Model of Actinosynnemapretiosum ATCC 31280 and Its Application for Ansamitocin P-3 Production Improvement. Genes (Basel) 9: 364.
- Li T, Fan Y, Nambou K, Hu F, Imanaka T, et al. (2015) Improvement of ansamitocin P-3 production by Actinosynnemamirum with fructose as the sole carbon source. Appl Biochem Biotechnol 175:2845-2856.
- Li Y, Kobayashi H, Hashimoto Y, Iwasaki S (1992) Binding selectivity of rhizoxin, phomopsin A, vinblastine, and ansamitocin P-3 to fungal tubulins: differential interactions of these antimitotic agents with brain and fungal tubulins. Biochem Biophys Res Commun 187:722-729.
- Finkelstein Y, Aks SE, Hutson JR, Juurlink DN, Nguyen P, et al. (2010) Colchicine poisoning: the dark side of an ancient drug. Clin Toxicol (Phila) 48:407-414.
- Kumar A, Sharma PR, Mondhe DM (2017) Potential anticancer role of colchicine-based derivatives: an overview. Anticancer Drugs 28:250-262.
- Ozen S, Kone-Paut I, Gul A (2017) Colchicine resistance and intolerance in familial mediterranean fever: Definition, causes, and alternative treatments. Semin Arthritis Rheum 47:115-120.
- Ozen S, Sag E, Ben-Chetrit E, Gattorno M, Gul A, et al. (2020) Defining colchicine resistance/intolerance in patients with familial Mediterranean fever: a modified-Delphi consensus approach. Rheumatology (Oxford).
- Wang TH, Wang HS, Soong YK (2000) Paclitaxel-induced cell death: where the cell cycle and apoptosis come together. Cancer 88:2619-2628.
- Weaver BA (2014) How Taxol/paclitaxel kills cancer cells. Mol Biol Cell 25:2677-2681.
- Yang YH, Mao JW, Tan XL (2020) Research progress on the source, production, and anti-cancer mechanisms of paclitaxel. Chin J Nat Med 18:890-897.
- Zhu L, Chen L (2019) Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett 24:40.











