Research Article - (2023) Volume 12, Issue 2
The Role of Exosome and Protein Kinase cGMP-dependent 1 (PRKG1) in Detecting of Breast Cancer in patients with Breast Tumors.
Nawfal K. Khiro1* and
Nadia N. Hasan2
1Dept. of surgery, college of medicine, Al- Nahrain University, Baghdad, Iraq
2Dept. of Basic Sciences, College of Dentistry, Ibn Sina University of Medical and Pharmaceutical Sciences, Baghdad, Iraq
*Correspondence:
Nawfal K. Khiro, Dept. of surgery, college of medicine, Al- Nahrain University, Baghdad,
Iraq,
Email:
Received: 30-Dec-2022, Manuscript No. Ipjbs-23-13357;
Editor assigned: 03-Jan-2023, Pre QC No. Ipjbs-23-13357;
Reviewed: 17-Jan-2023, QC No. Ipjbs-23-13357;
Revised: 24-Jan-2023, Manuscript No. Ipjbs-23-13357;
Published:
30-Jan-2023, DOI: 10.36648/2254-609X.23.12.01-94
Abstract
Background: Breast cancer is responsible for the death of millions of women worldwide every year. Several clinical, diagnostic and pathological techniques have been introduced to get early detection of the disease. One of these techniques was developed to detect extracellular vesicle exosomes and phosphoproteins as a product of cancer cells.
Objective: Investigating the role of exosome and (PRKG1) in detection breast cancer in patients with breast tumors.
Subjects and Methods: This case-control study was conducted on women. They were divided into 3 groups, group me of patients with malignant breast tumor, group II of patients with benign breast tumor, and group III which of matched healthy subjects as control. Ten milliliters of blood sample was obtained from patients submitted for surgery (mastectomy or lumpectomy) by venipuncture just before operation, they were collected during the period between Aprils -December 2018 in Al-Imamain Al-Kadhymeaain Medical City, Baghdad, Iraq. Plasma Exosomes and exosomal (PRKG1) were measured by ELIZA technique. Serum Cancer antigen 15-3 measured by ELIZA technique.
Results: There is a significant difference in the levels of exosomes and the (PRKG1) between breast cancer group and control group (p> 0.001). Also significant differences found between the breast cancer and benign group, and between benign and control group. In the concentration of antigen 15-3, a significant difference found between the breast cancer group control groups (p= 0.003), whereas there are no significant differences observed between the breast cancer and benign group (p=0.187) and between benign and control group (p=0.205).
Conclusions: Exosomes and exosomal (PRKG1) levels would be very good biomarkers for early detection and diagnosis of breast cancer and also can differentiate between malignant and benign breast tumors acting as a non-invasive liquid biopsy. Cancer antigen 15-3 marker has many limitations.
INTRODUCTION
Breast cancer is the most common cancer among women worldwide, comprising 23% of the 1.1 million female with cancers which newly diagnosed each year. In Iraq, breast cancer is the commonest type of malignancy in females and there is a general direction towards an increase in the frequency of breast cancer as well as increase incidence in younger age group, also breast cancer rates in Iraqi region were generally stable between 2000 to 2009, but newer data from the Iraqi Cancer Registry reveal rising rates since 2009 with women after age 50 making the major contribution to the increase incidence of breast cancer. Patients younger than 30 years old age formed about 5% of cases, whereas about 75% of the cases signed in women with women 40-60 years and the rest 20% represented women older than 60 years. The cause of breast cancer remains unknown, but numerous factors have been directly associated with increased risk of breast cancer incidence. Age, gander, personal history of breast cancer and a family history of breast cancer have the greatest relative factors [1].
Despite significant progress in breast cancer research, reliable biomarkers have yet to be identified. The current methods for early breast cancer detection, namely clinical examination and mammography, have certain limitations in their sensitivity and specificity. For example, mammography can detect only 70–90 % of breast lesions, with a false-positive rate of up to 31 %. Thus, accelerating progress against breast cancer requires both the development of novel biomarkers and therapeutic targets and further understanding of the potential molecular mechanisms [2].
Extracellular vesicles (EVs) are cell-derived Nano-vesicles, present in almost all types of body fluids, which play an important role in intercellular communication and are involved in the transport of biological signals for regulating diverse cellular functions. Cancer cells secrete a large number of exosomes compared with non-cancerous cells. Exosomes released from cancer cells stimulate cancer cells growth and mobility and the immune cells response for cancer progression and metastasis. So, Exosomes can be used as a source of biomarkers for, predicting metastatic tumors, targeted drug delivery, cell free antitumor vaccination and gene therapy. In breast cancer exosomes have been proved to regulate metastasis, stem cell stimulation, apoptosis, immune suppression, and drug resistance.
There are more than 100 exosomal phosphoproteins that may elevate in plasma of breast cancer patients. But the most significantly higher phosphoproteins in breast cancer patients compared with healthy controls are cGMP-dependent protein kinase 1 (PRKG1) and tight junction protein 2 (TJP2), which showed significant up regulation in breast cancer patients [3].
Materials and Method
A case control study was done on 70 women range from 40 -70 years who had breast tumor recruited from Operations Hall in general surgery Department at Al Imamain Al-Kathemeaain medical city, Baghdad, Iraq. Patients were divided into 2 subgroups, thirty-five newly diagnosed untreated women with malignant breast tumor [approved by histopatholgist and thirty-five newly diagnosed untreated women with benign breast tumor [approved by histopatholgist. Thirty-five healthy women were also included in this study as the normal controls (age range 40 -70 years) that have no family history of any type of cancer were involved in the study. The study was approved by the local Ethical Committee of the College of Medicine, Al-Nahrain University, and Baghdad, Iraq. In addition, an informed written consent for participation in the study was signed by the participant or the legal guardians of the investigated subjects according to the Helsinki principles. Women age more than 70 years and less than 40 years, or with tumors anywhere other than breast, Women have history of breast tumors, women on drugs or having comorbid disease such as liver, GIT, etc. and smoker women all of them were excluded from the study [4, 5].
Seven millilitres (7ml) of venous blood were withdrawn preoperatively from patients and controls into EDTA tubes. These tubes will be centrifuged at 12,000 rpm at room temperature for 30 minutes and then the Plasma obtained was kept in deep freeze (at −20°C) until the time of determination of Plasma exosomes and exosomal phosphoproteins (PRKG1 and TJP2).
Plasma exosomes were analysed by Human exosome 2 ELISA kit (Kono-Catalog no. KN1509Hu-china) while, exosomal phosphoproteins (PRKG1 and TJP2) determined by using Human PRKG1 ELISA Kit- Mybiosource Catalog no. MBS2504113- USA [6].
Statistical Analysis
The data of the study were stored in Microsoft excel spread sheet and analyzed on the computer using the SPSS software 20 and Microsoft excel program (2010). Numeric variables were expressed as mean ± SD and all statistical comparisons were made by means of independent t-test and ANOVA test with P ≤0.05 was considered statistically significant [7,8].
The correlation was done between all parameters using Pearson correlation test (Norman, 2010), all statistical analyses were carried out using SPSS program. Receiver Operating Characteristic (ROC) analysis was performed as a comprehensive way to assess the accuracy of the studied markers. The area under the curve (AUC) provides a useful tool to compare different biomarkers. The area under the curve (AUC) provides a useful tool to compare different biomarkers.
Results
Some demographic characteristics of the studied groups are summarized in Table 1. Non-significant differences in age and body mass index (BMI) were found among all studied groups Table 1.
| Variable |
Control |
Benign breast tumor |
Malignant breast tumor |
P-value |
| Number |
35 |
35 |
35 |
|
| Age |
45.92±2.59 |
47.28±2.89 |
49.7±3.2 |
0.74a |
| |
|
|
|
0.24b |
| BMI |
26.36±4.89 |
27.82±5.05 |
28.53±5.34 |
0.54a |
| |
|
|
|
0.15b |
Table 1. Demographic characteristics of the breast tumor patients and controls
Results in Table 2. revealed that there were non-significant differences in the levels of CA15-3 between females with benign breast tumor and control (p= 0.205) also between patients with benign and malignant breast tumor (p=0.187). On the other hand, a significant increase (p=0.003) in the level of CA15-3 in patients with malignant tumor was observed in a comparison with control subjects. Furthermore, ANOVA. Test revealed that there was a significant difference (p=0.004) among all studied groups as demonstrated in Table 2. In Table 2 the results showed that the levels of EXOSC2 increased significantly in females with benign tumor in comparison with controls (p=0.044) and increased further in patients with malignant breast tumor significantly when compared with either control (p <0.001) or benign tumor subjects (p <0.001).
| |
Group |
mean±SD |
Pa |
Pb |
Pc |
Pd |
| CA 15-3 (U/ml) |
Control |
|
0.205 |
0.003 |
0.187 |
0.004 |
| |
n= 35 |
22.38 ±7.26 |
|
|
|
|
| |
Benign tumor |
26.72±7.06 |
|
|
|
|
| |
n=35 |
|
|
|
|
|
| |
Malignant |
30.46±10.26 |
|
|
|
|
| |
Tumor |
|
|
|
|
|
| |
n=35 |
|
|
|
|
|
| EXOSC2 (ng/l) |
Control |
238.51±32.78 |
0.044 |
<0.001 |
<0.001 |
<0.001 |
| |
n= 35 |
|
|
|
|
|
| |
Benign tumor |
316.64±31.85 |
|
|
|
|
| |
n=35 |
|
|
|
|
|
| |
Malignant |
640.06±118.74 |
|
|
|
|
| |
Tumor |
|
|
|
|
|
| |
n=35 |
|
|
|
|
|
Table 2. CA15-3 and EXOSC2 levels in controls, patients with benign and malignant breast tumor
Levels of PRKG1 showed a similar pattern of a significant increase in patients with either benign or malignant breast tumor when compared with controls (p<0.001, p=0.001, respectively) as revealed in Table 3. Moreover, a significant increase in the level of PRKG1was observed in patient with malignant breast cancer in comparison with patients with a benign tumor (p<0.001) [9, 10].
| |
Group |
mean±SD |
Pa |
Pb |
Pc |
Pd |
| PRKG1 (ng/ml) |
Control |
2.14±0.41 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
| |
n= 35 |
|
|
|
|
|
| |
Benign tumor |
4.38±0.42 |
|
|
|
|
| |
n=35 |
|
|
|
|
|
| |
Malignant |
6.91±0.79 |
|
|
|
|
| |
Tumor |
|
|
|
|
|
| |
n=35 |
|
|
|
|
|
Table 3. PRKG1 levels in controls, patients with benign and malignant breast tumor
Correlations between the levels of the Studied Parameters among Control Subjects
According to results illustrated in Table 4 and Figure 1 there were significant positive correlations between EXOSC2 and PRKG1 (r=0.967; p<0.001).
| |
|
CA153 |
EXOSC2 |
PRKG1 |
| CA153 |
r |
1 |
0.118 |
-0.01 |
| |
p |
|
0.621 |
0.968 |
| EXOSC2 |
r |
|
1 |
.967** |
| |
p |
|
|
0 |
| PRKG1 |
r |
|
|
1 |
| |
p |
|
|
|
Table 4. Correlations between the levels of all studied parameters among control subjects 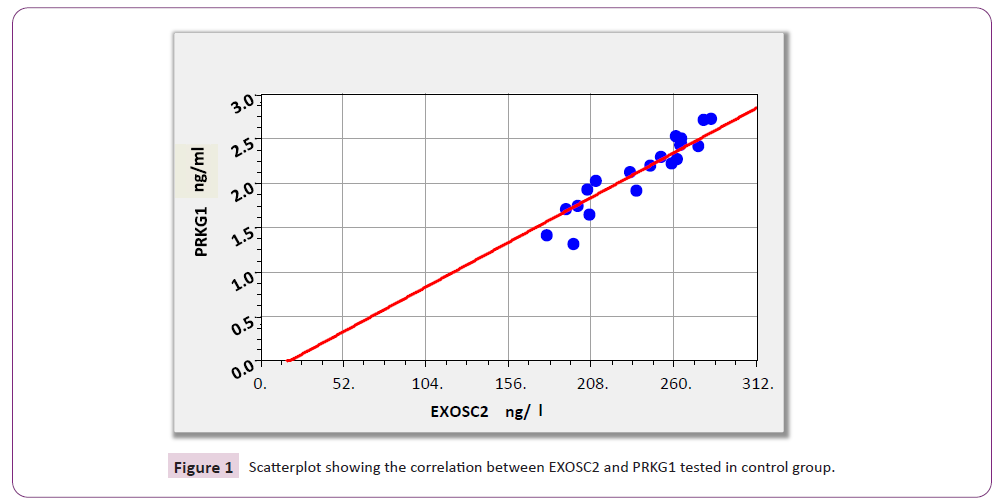
Figure 1: Scatterplot showing the correlation between EXOSC2 and PRKG1 tested in control group.
Correlations between the levels of the studied parameters among patients with benign breast cancer as demonstrated in Table 5 and Figure 2 EXOSC2 showed significant positive correlations with PRKG1 (r=0.869; p=0.031).
| |
|
CA153 |
EXOSC2 |
PRKG1 |
| CA153 |
r |
1 |
0.288 |
0.037 |
| |
p |
|
0.13 |
0.848 |
| EXOSC2 |
r |
|
1 |
0.869 |
| |
p |
|
|
0.031 |
| PRKG1 |
r |
|
|
1 |
| |
p |
|
|
|
Table 5. Correlations between the levels of all studied parameters among patients with benign breast tumor 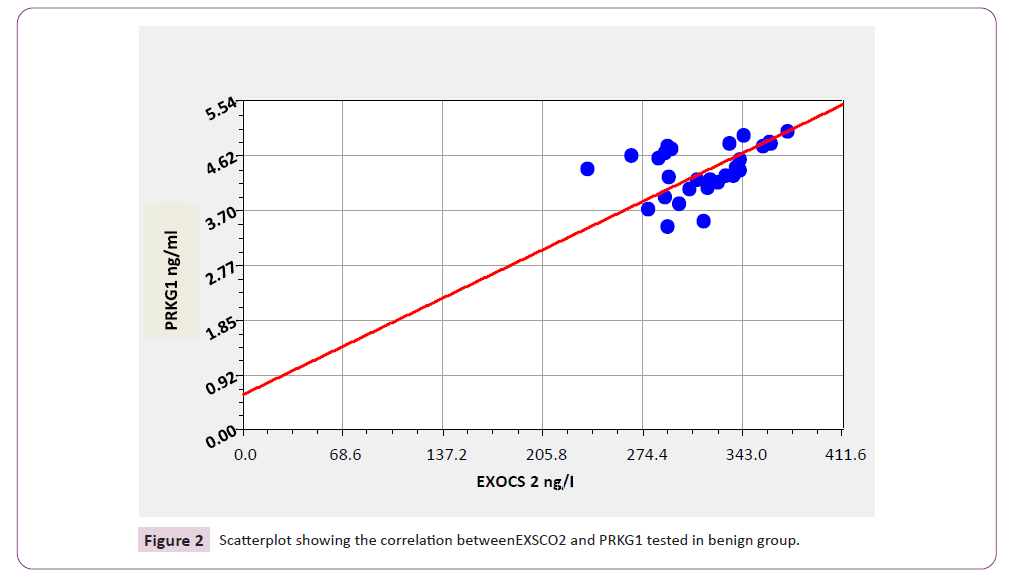
Figure 2: Scatterplot showing the correlation betweenEXSCO2 and PRKG1 tested in benign group.
Results in Table 6 and Figure 3 revealed that there is significant positive correlation between CA15-3 and all other tumor markers included in this study; EXOSC2, PRKG1 (r=0.665; p<0.001, and r=0.693; p<0.001 respectively), in addition to positive significant correlation between EXOSC2 and PRKG1 (r=0.944; p<0.001).
| |
|
CA153 |
EXOSC2 |
PRKG1 |
| CA153 |
r |
1 |
.665** |
.693** |
| |
p |
|
0 |
0 |
| EXOSC2 |
r |
|
1 |
.944** |
| |
p |
|
|
0 |
| PRKG1 |
r |
|
|
1 |
| |
p |
|
|
|
Table 6. Correlations between the levels of all studied parameters among patients with malignant breast tumor
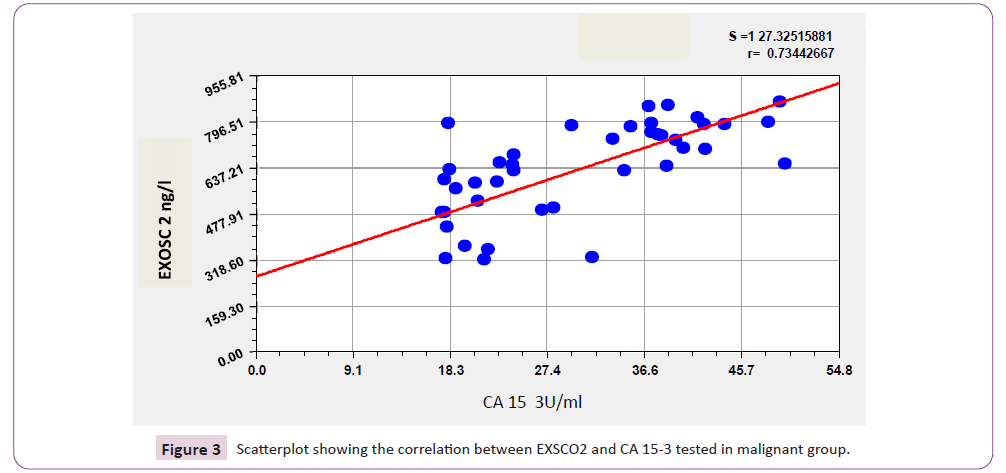
Figure 3: Scatterplot showing the correlation between EXSCO2 and CA 15-3 tested in malignant group.
Receiver Operating Characteristic (ROC) analysis for the studied parameters in patients with benign breast tumor comparing with controls.
According to results revealed in Table 7 and Figure 4 the area under the curve (AUC) of EXOSC2 and PRKG1 levels showed a high value (0.972 and 1.00 respectively) with excellent values of (90% and 100% respectively) in patients with benign breast tumor comparing with healthy controls.
| Parameters |
AUC |
Sensitivity (%) |
Specificity (%) |
Cut-off value |
| CA153 |
0.676 |
83 |
55 |
22.04 |
| EXOSC2 |
0.972 |
90 |
100 |
284.7 |
| PRKG1 |
1 |
100 |
100 |
3.08 |
Table 7. ROC curve results for all studied parameters in patients with benign breast tumor comparing with controls 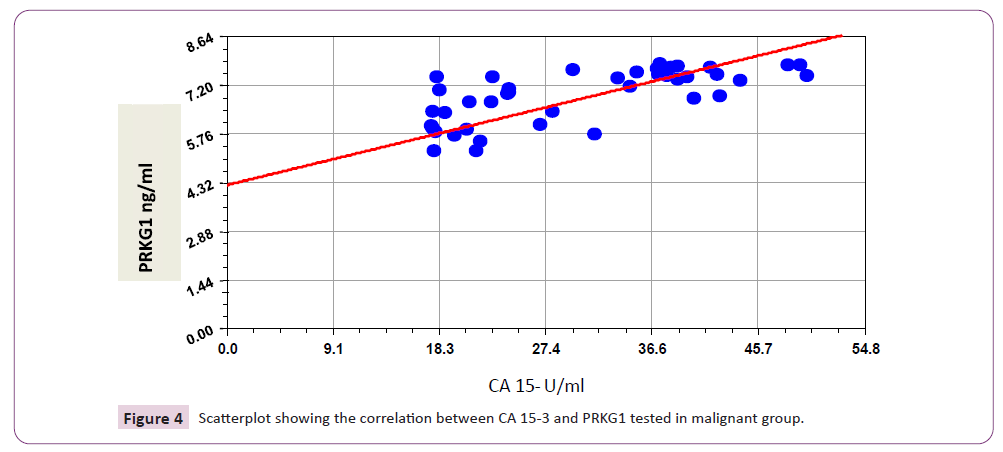
Figure 4: Scatterplot showing the correlation between CA 15-3 and PRKG1 tested in malignant group.
ROC curve results in Table 8 and Figure 5 showed that the area under the curve (AUC) of EXOSC2, and PRKG1 levels had a high value (0.964, and 1.00 respectively) with excellent values of specificity (97%, 100% respectively) and sensitivity (90% and 100% respectively) in patients with malignant breast tumor comparing with benign breast tumor patients.
| Parameters |
AUC |
Sensitivity (%) |
Specificity (%) |
Cut-off value |
| CA153 |
0.59 |
46 |
83 |
29.63 |
| EXOSC2 |
0.964 |
90 |
97 |
411.03 |
| PRKG1 |
1 |
100 |
100 |
5.15 |
Table 8. ROC curve results for all studied parameters in patients with malignant breast tumor comparing with benign breast tumor patients 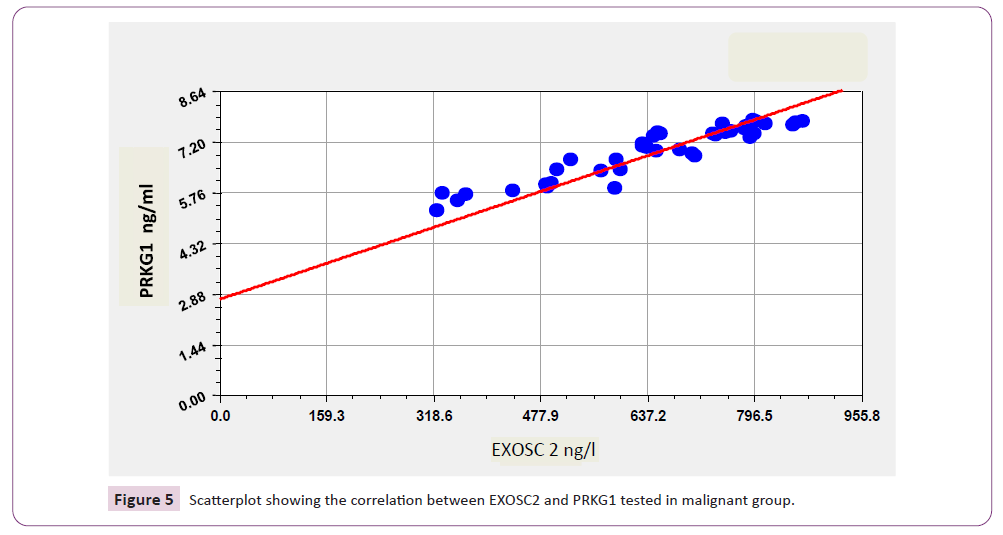
Figure 5: Scatterplot showing the correlation between EXOSC2 and PRKG1 tested in malignant group.
Results obtained in Table 9 revealed that the area under the curve (AUC) of EXOSC2 and PRKG1 levels showed a high value (1.00 for both of them) with excellent values of specificity and sensitivity (100% for both) in patients with malignant benign breast tumor comparing with controls.
| Parameters |
AUC |
Sensitivity (%) |
Specificity (%) |
| CA153 |
0.748 |
78 |
55 |
| EXOSC2 |
1 |
100 |
100 |
| PRKG1 |
1 |
100 |
100 |
Table 9. ROC curve results for all studied parameters in patients with malignant breast tumor comparing with controls
Discussion
Exosomes have been proven to be important regulators in disease state, especially in tumor biology. Tumor derived exosomes contains tumor specific antigens and nucleic acids can be assessed as a non-invasive potential diagnostic and predictive biomarker for the early diagnose of breast cancers and for evaluation of therapeutic effect and patient’s outcome.
Results in Table 2 showed that patients with breast cancer involved in this study have a significantly higher circulating plasma exosome level (p<0.05) compared to nearly comparable age- and sex matched control subjects which is in agreement with previous studies that demonstrated a significantly higher plasma exosomal levels in breast cancer patients in comparison with control subjects.
Jia et al., 2017 reviewed that breast cancer cells release greater amount of exosomes into circulation which could be isolated from serum, pleural effusions, urine, and ascites fluids of breast cancer patients, so alterations in expression of exosomal levels from body fluids of cancer patients propose the role for exosomes as an early marker for diagnosis, prognosis and monitoring of breast cancer patients.
Moreover, the results obtained from Table 2 showed a high significant level of plasma exosome (p<0.05) in breast cancer patients when compared with nearly comparable age- and sex matched patients having benign breast tumors. These results comparable with those presented previously. Results of the current study revealed that there were significant differences in the levels of plasma exosome between patients with benign breast tumors and matched control subjects. So, according to these results exosomes can be considered as a powerful breast cancer biomarker which can act as a non-invasive liquid biopsy that differentiates malignant from benign breast tumors. This is greatly supported, which demonstrated that exosome collected from body fluids of cancer patients give full information about tumor type, size and site of tumor in body similar to that obtained by surgical biopsy. Also explain that Circulating EVs represented by exosomes have been included in the novel concept of “Liquid Biopsies” as the most reliable prognostic and diagnostic tools for both screening and early diagnosis of cancer and cancer recurrence.
Additionally, Li et al., 2017 illustrated that the exosome level in pre-clinical data was highly correlated with the tumor mass which greatly support the hypothesis that plasmatic levels of EVs (including exosomes) may represent a valuable tumor marker. Therefore, previous study revealed that exosomes can be measured earlier than biopsy, MRI or breast x ray to detect breast cancer. The Receiver Operating Characteristic (ROC) curve in Table 7, Table 8, and Table 9 showed that Exosomes had very high sensitivity and specificity values that prove its importance in detecting early stage of breast cancer and benign tumor beside its role in differentiating malignant tumor from benign one. So these results suggested that Exosomes can be used as sole marker that predict and differentiate breast cancer from other benign or non-breast diseases. These results were in agreement with several previous studies that assumed the crucial role of exosome in breast cancer detection.
Cyclic- Gunalyl Mono Phosphate Dependent Protein Kinase 1 (PRKG1)
Cyclic GMP-dependent protein kinase 1 (PRKG1) signalling pathways exert an important effect on the growth of cancer cells. The evidence is that that PRKGI plays a direct role in the growth of breast cancer tumors. The present study showed that the mean and standard deviation of PRKG1 in sera of malignant, benign breast tumor's women and control group were (6.91±0.79; 4.38±0.42; and 2.14±0.41 ng/ml respectively), which indicates that there were highly significant elevation in PRKG1 levels in breast cancer group as compared with benign and control groups (p< 0.001) which is in agreement with who demonstrated that exosomeal phosphoproteins(specially PRKG1 ) in plasma of breast cancer cases clearly presented with a predictable high concentrations that can provide a useful real-time information in the early detection and monitoring of breast cancer.
Also results in Table 3 showed that PRKG1 levels were significantly elevated in benign group as compared with control one (p< 0.001), and this results supported by studies accomplished by the several researchers. From the above explanations PRKG1 in addition to other parameters tested in this study can be used as a powerful tumor marker to demonstrate and differentiate the type, size and stages of breast tumors, these finding greatly supported by.
In addition to the above mentioned data, Receiver Operating Characteristic (ROC) curve results in Table 7, Table 8, and Table 9 also indicate that PRKG1 can be considered as an excellent parameter for the diagnosis and for differentiation of breast tumors in agreement with the previously mentioned studies. ROC curve results showed that PRKG1 level had high sensitivity and specificity (100%, 100% respectively) with a high area under the curve AUC (nearly 1) values which prove its valuable role in detecting breast cancer.
Cancer Antigen (CA 15-3)
Carcinoma Antigen is a tumor marker for many types of cancer, most notably breast cancer widely used for many years. Even it does not play great role in screening for primary breast cancer, but its more useful in follow-up care.
Results in Table 2 showed that the levels of CA 15-3 in women with breast cancer subjected to this study were significantly higher than that in nearly comparable age- and sex matched control subjects (p<0.005) which is in agreement with previous research conducted by Zale ski and his colleagues that revealed a significant higher CA 15-3 level in women with malignant breast tumor.
The results of Table 3 also showed that there were non-significant differences in CA 15-3 levels (p>0.005) between patients undoubtedly have breast cancer and those matched comparable (age / sex) patients proven to have benign breast tumor which is in consistency with results obtained study who found that tumor marker CA 15-3 in breast cancer patients were non-significantly differ from that obtained in women with benign breast tumor. They also reviewed that CA15-3 levels considered as valuable tumor marker only in late stages of breast cancer and support therapy response assessment and detection of recurrent disease. On the other hand, CA15-3 levels sensitivity showed to be limited in early stages of breast cancer as revealed by previous literature. Furthermore, this finding was typically supported by Felix et al., who postulated that there was about (30%-60%) of patients with benign breast tumor presented with increased concentration of pre-treatment CA 15-3 that give mixed feelings on the diagnostic utility of this marker.
Moreover, results obtained in the current study revealed that there was a non-significant difference in CA 15-3 levels between patients with benign breast tumor and control subjects. This result greatly agreed with study conducted by Duffy and his coworkers which demonstrated that there is high percentage of healthy women having higher levels of CA 153 in their circulation due to non-cancerous causes such as colon, liver, lung, pancreatic, ovarian and prostate that can produce CA 15- elevation. Similarly, certain benign diseases such as chronic active hepatitis, liver cirrhosis, sarcoidosis, hypothyroidism and megaloblastic anemia may increase CA 15-3 levels, although in these situations the increases are usually modest.
The Receiver Operating Characteristic (ROC) curve results revealed in Table 7 show that CA 15-3 levels had moderate sensitivity and low specificity in detection and diagnosis of early stage breast cancer so we can't relies greatly on this marker alone to predict and differentiate breast cancer from other benign or non-breast diseases that is in agreement with many previous studies.
Correlations between all Studied Tumor Markers
Correlation between Studied Tumor Markers in Control Group
In the current study, the level of EXOCS2 showed a highly significant positive correlation with the levels of PRKG 1 and TJP2 as revealed in Table 4. This finding also prove the relationsh between these markers even in healthy subjects which may provide a promising result that can be used in future for the early diagnosis and also prognosis of many related problems such as benign and malignant breast tumor.
On the other hand, the results obtained in this study revealed that the levels of Ca 15-3 were non-significantly correlated with other studied parameters that might be owned to the fluctuation in the levels of CA 153 in a manner independent from other studied parameters which may be caused by the elevation in their levels as a result of many other noncancerous conditions as demonstrated in several previous studies.
Correlation between Studied Tumor Markers in benign Group
The results of this study ensure the previous findings which suggest that the EXOSC2, and PRKG1 levels were significantly correlated with each other that prove the possibility of using these biomarkers as a tool for diagnosis of both malignant and benign breast tumor either alone or in a combination as they increased in a parallel manner that revel the strong relationship among these markers which can be established in future as a powerful indicator for the diagnosis and differentiation between heathy women and women with either benign or malignant tumor. Chen and his co-workers’ opinion were in consistency with results obtained by the current study in which they found that the levels of the studied markers can be used in a differentiation between patients with breast cancer and those with benign breast mass.
In this study, CA 15 -3 levels in women with benign breast tumors showed non-significant correlations with studied parameters (exosomes, and PRKG1) as seen in Table 5 that it might be caused by the increment in these levels in patients that have benign breast tumor for prolonged time while women that diagnosed early with the benign mass showed low CA15-3 levels, So, collectively the levels of this parameter is not sensitive enough to differentiate the benign breast tumor women from those with malignant tumor or also differentiate them from the healthy subjects. This finding is comparable with that obtained previously by Liang and others in 2018 who postulated that the levels of CA15-3 were falsely elevated in several non-cancerous conditions which reduce the selectivity of this tumor marker in diagnosis.
Correlation between Studied Tumor Markers in Malignant Group
In the current study, we noticed previously a highly significant increase in the levels of all studied parameters in the malignant group in a parallel pattern that appear more clearly in the positive significant correlations demonstrated in Table 6 which the level of EXOSC2 showed a very highly significant positive correlation with the levels of PRKG 1.
The results of parameters that showed high levels with highly significant positive correlations can be used to predict and demonstrate the presence of malignant breast mass and differentiate it from unknown breast tumors without the need for an invasive surgical biopsy in consistency with previous studies which assumed that EXOSC2 released actively by cancer cell and can be used as an alternative for the commonly used invasive methods. Furthermore, mentioned that traditional biopsies, such as fine needle aspiration, rely on accessing the tumor cells, but exosome-based liquid biopsy relies on subcellular particles and their cargos. Circulating tumor cells thought to be a new source for cancer biomarkers. Exosomes are stable in the circulation and are found in almost every potential body fluid; therefore, they can be used as diagnostic tools for many diseases including breast cancer. Additionally, the levels of CA 15-3 in malignant conditions showed positive correlation with other studied parameters as it increase to high level (above 30 u/ml) in breast cancer group compare with benign and control groups which is in consistency with previous literature presented by Geng and his colleagues. These findings are very important since it provide the clinicians with a new approach for the diagnosis of breast cancer using a combination of the above studied tumor markers that improve their diagnostic and differentiating capability in an attempt to get the most powerful way for establishment of tumor diagnosis system.
Conclusion
Exosomes and exosomal phosphoproteins Cyclic guanosine mono- phosphate -dependent protein kinase1 and Tight junction protein 2 levels were showed to be very good biomarkers for early detection and diagnosis of breast cancer. In addition, they can differentiate between malignant and benign breast tumors and act as a non-invasive liquid biopsy. Cancer antigen 15-3 as a currently used tumor marker has many limitations with low specificity and can't depend greatly on it in diagnosis of breast cancer.
References
- Queiroz-Telles F, Fahal AH, Falci DR, Caceres DH, Chiller T, et al. (2017) Neglected endemic mycoses. The Lancet. Infectious Diseases 17: 367-377.
Indexed at, Google Scholar, Crossref
- Seyedmousavi S, Bosco SM, de Hoog S, Ebel F, Elad D, et al. (2018) Fungal infections in animals: a patchwork of different situations. Medical Mycology 56: 165–187.
Indexed at, Google Scholar, Crossref
- Sehgal M, Ladd HJ, Totapally B (2020) Trends in Epidemiology and Microbiology of Severe Sepsis and Septic Shock in Children. Hospital Pediatrics 10: 1021-1030.
Indexed at, Google Scholar, Crossref
- Song G, Liang G, Liu W (2020) Fungal Co-infections Associated with Global COVID-19 Pandemic: A Clinical and Diagnostic Perspective from China. Mycopathologia 185: 599-606.
Indexed at, Google Scholar, Crossref
- Nwokolo NC, Boag FC (2000) chronic vaginal candidiasis Management in the postmenopausal patient. Drugs & Aging 16: 335-9.
Indexed at, Google Scholar, Crossref
- Akpan A, Morgan R (2002) Oral candidiasis. Postgrad Med J 78: 455-9.
Google Scholar, Crossref
- Lippi D, Gotuzzo E (2014) the greatest steps towards the discovery of Vibrio cholerae. Clin Microbiol Infect 20: 191-195.
Indexed at, Google Scholar, Crossref
- Jugder BE, Batista JH, Gibson JA, Cunningham PM, Asara JM, et al. (2022) Vibrio cholerae high cell density quorum sensing activates the host intestinal innate immune response. Cell Reports 40: 111368.
Indexed at, Google Scholar, Crossref
- Jugder BE, Watnick PI (2020) Vibrio cholerae Sheds Its Coat to Make Itself Comfortable in the Gut. Cell Host & Microbe 27: 161-163.
Indexed at, Google Scholar, Crossref
- Song Tianyan, Mika Franziska, Lindmark Barbro, Schild Stefan, Bishop Anne, et al. (2008) A new Vibrio cholerae sRNA modulates colonization and affects release of outer membrane vesicles. Molecular Microbiology 70: 100-111.
Indexed at, Google Scholar, Crossref
Citation: Khiro NK, Hasan NN (2023) The
Role of Exosome and Protein Kinase cGMPDependent
1 (PRKG1) in Detecting of Breast
Cancer in Patients with Breast Tumors. J
Biomed Sci, Vol. 12 No. 1: 94










