Kaan Meric, Ceren Yalniz*, Hasan Gundogdu, Sibel Aydin, Zeynep Gamze Kilicoglu and Mehmet Masum Simsek
Haydarpasa Numune Training and Research Hospital of Radiology, Tibbiye, Uskudar/Istanbul, Turkey
Corresponding Author:
Ceren Yalniz
Haydarpasa Numune Training and Research Hospital Radiology, Tbbiye Cad. No: 23 34668 Uskudar/ Istanbul, Turkey
Tel: 00905057084210
Fax: 00902163360565
E-mail: cerenyalniz@gmail.com
Received date: May 19, 2016; Accepted date: June 27, 2016; Published date: June 30, 2016
Introduction: Although MRI is the primary modality in the diagnosis of cranial metastasis, advanced MRI techniques such as spectroscopy, perfusion, diffusion weighted imaging, and diffusion tensor imaging are used to distinguish cranial metastasis from other pathologies. Since cranial metastasis are often highly vascular lesions, on perfusion examination they tend to exhibit elevated cerebral blood volume (rCBV). However since high-grade glial tumors also exhibit elevated rCBV, perfusion imaging cannot accurately differentiate between these two groups. Peritumoral rCBV values are actually more reliable in differential diagnosis of multicentric glial tutors and cranial metastasis.
Case report: A fifty-six-year-old male presented with progressive weakness in his left arm. MRI revealed multiple, different-sized lesions with a wide range of intensity changes. Some of the lesions displayed substantial peritumoral vasogenic edema. On DWI sequences, lesions demonstrated peripheral restricted diffusion. MRP sequences didn't show elevated rCBV values in T2-hyperintense peritumoral edema areas. The excisional biopsy of the lesion in left occipital lobe performed and the lesion's pathology result was reported as small-cell lung carcinoma metastasis, supporting our primary radiologic diagnosis.
Discussion: Advanced MRI techniques such as perfusion, spectroscopy, diffusion-weighted imaging (DWI) and diffusion tensor imaging (DTI) are used in the context of distinguishing cranial metastasis from high-grade glial tumors. Perfusion parameters like rCBV and rCBF are important to distinguish metastatic lesions from cranial lymphoma and benign cranial masses like abcess. However, since both cranial metastasis and high-grade glial lesions are both hypervacular, comparison of rCBV of the enhancing component of lesions cannot differentiate between these two groups. In such cases, rCBV values obtained from the peritumoral T2 hyperintense edema component of the lesion can help us. Conclusion: Multicentric glial tumors are on the differential diagnosis list of cranial metastasis. When conventional MRI techniques are insufficient to differentiate these two entities, perfusion values obtained from the peritumoral edema areas might be helpful.
Keywords
Cranial metastasis; Magnetic resonance perfusion; rCBV; rCBF
Introduction
Although magnetic resonance imaging (MRI) is the primary modality in the diagnosis of cranial metastasis, advanced MRI techniques such as magnetic resonance spectroscopy (MRS), magnetic resonance perfusion (MRP), diffusion weighted imaging (DWI), and diffusion tensor imaging (DTI) are used to distinguish ambiguous cranial metastasis from other pathologies. Since cranial metastasis are often highly vascular lesions, on MRP examination they tend to exhibit elevated cerebral blood volume (rCBV) compared with contralateral normal white matter. However since high-grade glial tumors also exhibit elevated rCBV, perfusion imaging cannot accurately differentiate between these two groups. Peritumoral rCBV values are sometimes more reliable in differential diagnosis of multicentric glial tumors and cranial metastasis.
Case Report
A fifty-six-year-old male presented with progressive weakness in his left arm. Cranial computed tomography (CT) scans revealed multiple cerebral and cerebellar hypodense cystic lesions. The biggest one on the right hemisphere was 18 × 20 mm, located subcortically in the parietal lobe and the biggest one on the left hemisphere was 40 × 42 mm, located cortically and subcortically in the occipital lobe.
MRI revealed infra/supratentorially located multiple, different-sized lesions with a wide range of intensity changes on T1 (Figure 1) and T2-weighted images (Figure 2). Some of the lesions displayed substantial peritumoral edema. On DWI sequences, lesions demonstrated peripheral restricted diffusion (Figure 3).
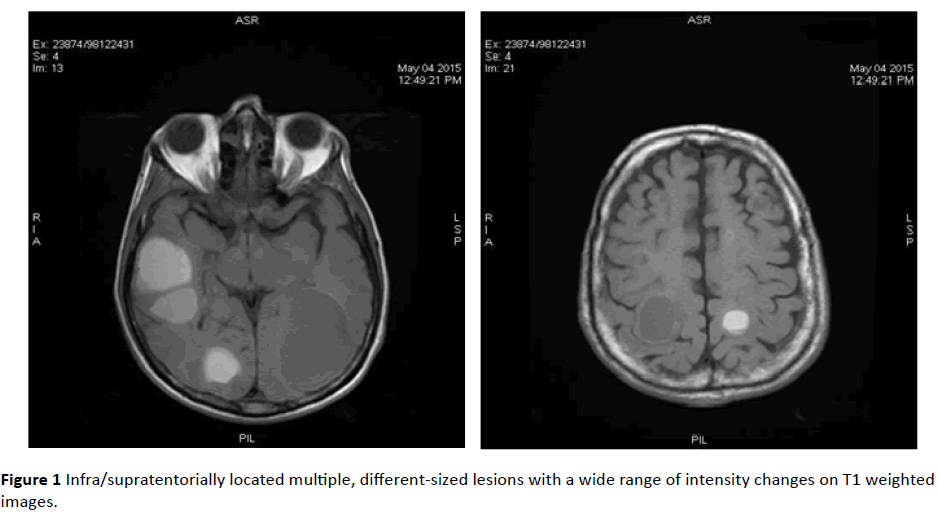
Figure 1: Infra/supratentorially located multiple, different-sized lesions with a wide range of intensity changes on T1 weighted images.
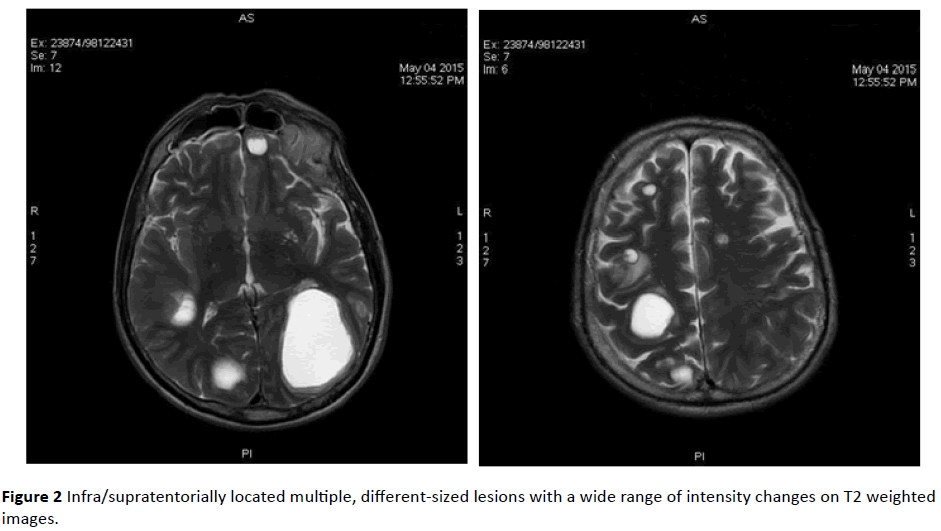
Figure 2: Infra/supratentorially located multiple, different-sized lesions with a wide range of intensity changes on T2 weighted images.
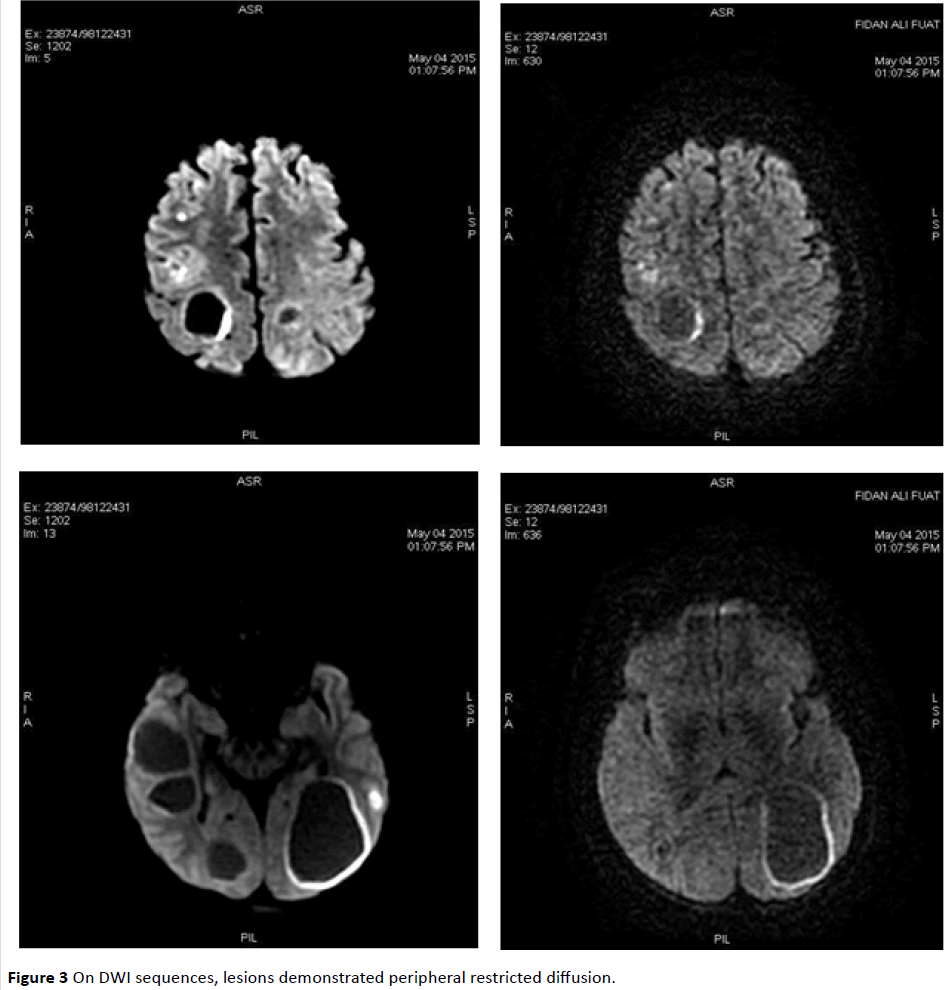
Figure 3: On DWI sequences, lesions demonstrated peripheral restricted diffusion.
MRP sequences didn’t show elevated rCBV and cerebral blood flow (rCBF) values in T2-hyperintense peritumoral edema areas, compared with contralateral normal white matter.
Maximum rCBV values were 3.94 in the peritumoral area of the left occipital lesion and 3.09 in the contralateral normalappearing white matter (Figure 4). Maximum rCBV value obtained from the peritumoral area around the right parietal lesion was 2.19 whereas rCBV value of the contralateral normal-appearing white matter was 2.64 (Figure 5).
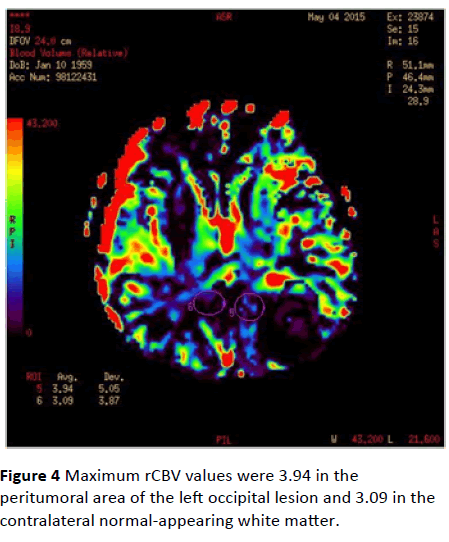
Figure 4: Maximum rCBV values were 3.94 in the peritumoral area of the left occipital lesion and 3.09 in the contralateral normal-appearing white matter.
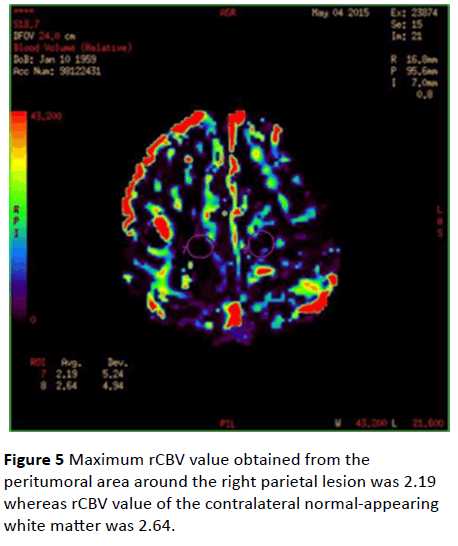
Figure 5: Maximum rCBV value obtained from the peritumoral area around the right parietal lesion was 2.19 whereas rCBV value of the contralateral normal-appearing white matter was 2.64.
Maximum rCBF values were 9.05 in the peritumoral area of the left occipital lesion and 9.17 in the contralateral normalappearing white matter (Figure 6). Maximum rCBF value obtained from the peritumoral area around the left occipital lesion was 4.80 whereas rCBV value of the contralateral normal-appearing white matter was 6.56 (Figure 7).
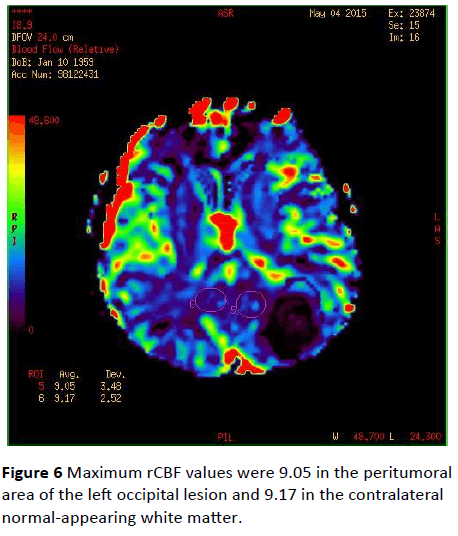
Figure 6: Maximum rCBF values were 9.05 in the peritumoral area of the left occipital lesion and 9.17 in the contralateral normal-appearing white matter.
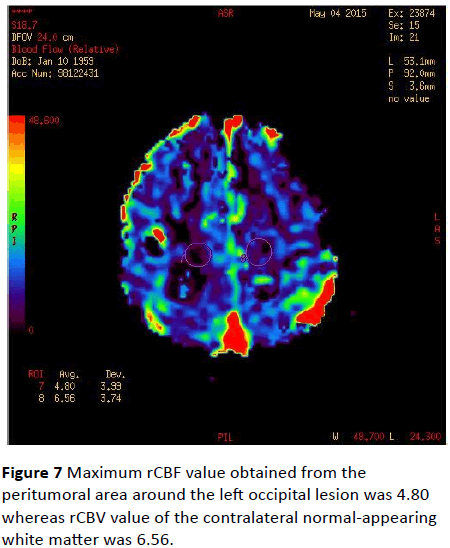
Figure 7: Maximum rCBF value obtained from the peritumoral area around the left occipital lesion was 4.80 whereas rCBV value of the contralateral normal-appearing white matter was 6.56.
DTI sequences showed that the lesions pushed the adjacent tracts without any sign of invasion (Figure 8). Since the patient didn’t cooperate with the investigation, MRS imaging couldn’t be performed.
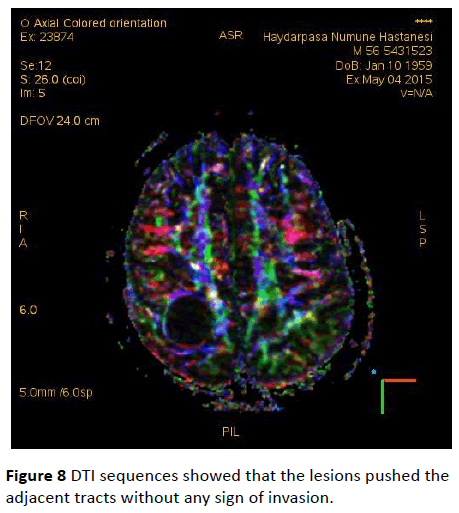
Figure 8: DTI sequences showed that the lesions pushed the adjacent tracts without any sign of invasion.
The excisional biopsy of the lesion in left occipital lobe was performed and the lesion’s pathology result was reported as small-cell lung carcinoma metastasis, supporting our primary radiologic diagnosis.
Discussion
MRI is the primary modality used for the detection of cranial metastasis. However, conventional MRI is usually insufficient for the differentiation between high-grade glial tumors and cranial metastasis. Conventional MRI sequences provide information about the anatomic and morphological aspects of the lesions. Chemical composition, characteristics and effects in the adjacent tissues are important to distinguish metastasis from glial tumors. These aspects can only be generated by advanced MRI sequences.
Advanced MRI techniques such as perfusion, spectroscopy, diffusion-weighted imaging (DWI) and diffusion tensor imaging (DTI) are used in the context of distinguishing cranial metastasis from high-grade glial tumors [1]. In perfusion imaging the volume of blood passing through a portion of the brain is measured. In other words perfusion-weighted imaging provides noninvasive measurements of tumor vascularity and identifies areas of neovascularization. As a brain tumor outgrows its blood supply, it induces angiogenesis; as a result, perfusion values are elevated.
Perfusion parameters like rCBV and rCBF distinguish metastatic lesions from cranial lymphoma and other benign cranial masses such as abcess. Whereas cranial metastasis show elevated perfusion parameters as a result of high vascularisation due to tumoral neoangiogenesis, abcess demonstrates decreased rCBV and lymphoma demonstrates lower rCBV values compared to glial tumors and metastasis [2]. However, since both cranial metastasis and high-grade glial lesions are both hypervacular, comparison of rCBV of the enhancing component of lesions cannot differentiate between these two groups [3-5]. In such cases, rCBV values obtained from the peritumoral T2 hyperintense edema component of the lesion can help us. Peritumoral signal represents interstitial water due to blood-brain barrier breakdown and capillary permeability changes. At pathologic examination, the peritumoral areas of primary high-grade gliomas contain infiltrating tumor cells in addition to interstitial water. However, the peritumoral area of metastasis doesn’t demonstrate tumoral infiltration [6,7]. Due to infiltrative nature of high-grade glial neoplasms, peritumoral rCBV values in glial tumors are higher than the peritumoral rCBV values of cranial metastasis. The peritumoral hypoperfusion in metastases is thought to be due to absence of tumor cells, mass effect of vasogenic peritumoral edema and cerebral blood flow reduction in edematous areas [8,9].
Conclusion
Multicentric glial tumors are on the differential diagnosis list of cranial metastasis. When conventional MRI techniques are insufficient to differentiate these two entities, perfusion values obtained from the peritumoral edema areas might be helpful.
9771
References
- Fink KR, Fink JR (2013) Imaging of brain metastases. Surgical Neurology International 4: S209-S219.
- Wetzel SG, Cha S, Johnson G (2002) Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology 224: 797–803.
- Bendini M, Marton E, Feletti A, Rossi S, Curtolo S, et al. (2011) Primary and metastatic intraaxial brain tumors: prospective comparison of multivoxel 2D chemical-shift imaging (CSI) proton MR spectroscopy, perfusion MRI, and histopathological findings in a group of 159 patients. ActaNeurochir (Wien) 153: 403–412.
- Calli C, Kitis O, Yunten N, Yurtseven T, Islekel S, et al. (2006) Perfusion and diffusion MR imaging in enhancing malignant cerebral tumors. Eur J Radiol 58: 394–403.
- Wong JC, Provenzale JM, Petrella JR (2000) Perfusion MR imaging of brain neoplasms. AJR Am J Roentgenol 14: 1147–1157.
- Meng Law, Soonmee Cha, Edmond AK, JohnsonG, ArnettJ, et al. (2002) High-grade gliomas and solitary metastases: Differentiation by using perfusion and proton spectroscopic MR imaging. Radiology 222: 715-721.
- Burger P (1990) Classification, grading, and patterns of spread of malignant gliomas. In: Apuzzo ML, (ed). Neurosurgical topics: malignant cerebral glioma. Park Ridge, Ill: American Association of Neurological Surgeons 3-17.
- Chiang IC, Kuo YT, Lu CY, Yeung KW, Lin WC, et al. (2004) Distinction between high-grade gliomas and solitary metastases using peritumoral 3-T magnetic resonance spectroscopy, diffusion, and perfusion imaging. Neuroradiology 46: 619-627.
- Sugo N, Harada N, Yokota K, Miyazaki C, Ohtsuka T, et al. (2003) Determination of peritumoralhypoperfusion volumes surrounding brain tumors using three-dimensional spect. CI Kenkyu 25: 77-85.













