Research Article - (2023) Volume 15, Issue 2
The trans-differentiation of Keratinocyte: Requisite for skin wound healing and cosmetic surgery
Dr. Mamata Mishra*
Senior Research Scientist, Jai Research Foundation, Research & Development Section, JRF Global, Valvada, Gujarat-396105, India
*Correspondence:
Dr. Mamata Mishra, Senior Research Scientist, Jai Research Foundation, Research & Development Section, JRF Global, Valvada, Gujarat-396105,
India,
Tel: +918291213057,
Email:
Received: 30-Jan-2023, Manuscript No. ijddr-23-13463;
Editor assigned: 02-Feb-2023, Pre QC No. ijddr-23-13463;
Reviewed: 16-Feb-2023, QC No. ijddr-23-13463;
Revised: 18-Feb-2023, Manuscript No. ijddr-23-13463;
Published:
25-Feb-2023, DOI: 10.36648-0975-9344-15.2-997
Abstract
Objective: Skin wound healing involves the interaction of epidermal and dermal
cells, growth factors, and cytokines. Keratinocyte is the most dominant cell type
in the epidermis and plays multiple roles for skin repair function. Cultured human
primary keratinocytes are required for the treatment of burns, cutaneous wounds
diabetic foot ulcers, and cosmetic therapy
Methods: Human primary keratinocytes from different age groups were isolated,
characterized and efficacy was analyzed. Primary human keratinocytes were
purchased from commercially available sources and their trans-differentiation
properties were studied.
Results: Serum-free media with high calcium was used to isolate keratinocytes
from different donors with varied age groups. The number of keratinocytes isolated
from the total population of cells varied depending upon the age. Periodical
photographs were being taken for analysis. Quantitative analysis of percentage
expression of CK5 and CK10 has been done in keratinocytes and fibronectin and
collagen staining has been done in transdifferentiated fibroblast.
Conclusion: Isolation of primary keratinocytes brings the hope that healthy cultured
keratinocytes are useful for the wound microenvironment. Trans-differentiation of
keratinocytes to fibroblast provides a new angle for understanding the interaction
between keratinocytes and fibroblast in cosmetic surgery and wound healing.
Keywords
Skin wound healing; Trans-differentiation; Cultured keratinocytes;
Primary human keratinocytes; Dermal fibroblast
Introduction
Advanced treatment strategies for skin regeneration are the first
and foremost step of cutaneous wound healing. Keratinocyte
therapy is the integral component of cell-based therapy for burn
wounds and other wounds in which cellular material, keratinocyte
injected, grafted or implanted into patient for healing of wound
repair. Transdifferentiation is the process in which one type of
adult tissue undergoes a phenotypic switch and differentiates
it from another type of functional adult tissue. This is caused
by a change in the expression of a master switch gene and the
cells exhibit the functional characteristics. The skin wound
repair varies upon the balanced process of cell proliferation
and differentiation of epidermal stem cells, keratinocytes and fibroblasts [1]. Advances in medical treatments have facilitated
survival rates of severe patients who face difficulties in healing the
wound. Primary keratinocytes in the form of cultured epithelial
autografts (CEA) were used clinically for the first time in the early
1980s. Since 1984 good results have been achieved while using
CEA by several groups. During the process of normal wound
healing, the skin allows repairing itself; however extensive burn
patients, diabetic ulcers, and road accidents require intervention
for tissue restoration. Especially, the role of Keratinocyte in
donor site healing and deep burn wound healing is a distinctive
event. Several research groups used keratinocyte cells to induce
the regenerative capacity of residual cells at the wound surface.
Cultured epithelial autograft (CEA) are aseptically processed
wound dressings, approved by FDA in 2007 [2]. The average fibroblasts [1]. Advances in medical treatments have facilitated
survival rates of severe patients who face difficulties in healing the
wound. Primary keratinocytes in the form of cultured epithelial
autografts (CEA) were used clinically for the first time in the early
1980s. Since 1984 good results have been achieved while using
CEA by several groups. During the process of normal wound
healing, the skin allows repairing itself; however extensive burn
patients, diabetic ulcers, and road accidents require intervention
for tissue restoration. Especially, the role of Keratinocyte in
donor site healing and deep burn wound healing is a distinctive
event. Several research groups used keratinocyte cells to induce
the regenerative capacity of residual cells at the wound surface.
Cultured epithelial autograft (CEA) are aseptically processed
wound dressings, approved by FDA in 2007 [2]. The average
Vol. 15 No. 2: 997
2023
Vol. 15 No. 2: 997
2
International Journal of Drug Development and Research
0975-9344
culture time of epithelial cells has been improved and the longtime
interval between biopsy and grafting has been reduced as a
result of which cells derived from a small biopsy have been used
for enhancement in the speed of re-epithelialization. The main
advantage of cultured epithelial autografts is that large burn
wounds can be covered with autologous cells derived from a small
biopsy. In terms of cosmetic results also, the improvement in the
speed of re-epithelialisation has been reported by various groups
and CEA seems to have better results when compared to widely
meshed autograft. However, the greatest merit of CEA is that it
harnesses the potential for grafting of epidermal keratinocytes
and it has preserved sufficient proliferative capacity. Taken
together, the keratinocyte-based treatment is promising, and it
holds the future impact of keratinocyte-mediated cell coverage
options, upon which is promising, but more research is needed [3].
Additionally, keratinocyte-based treatments need to be pursued
carefully, as the other side of the story invites hypertrophic
scarring if over-activation of keratinocytes can subsidize. As
the cell culture is an expensive process and the cost-benefit
relationship of this method is comprehensively discussed and the
potential of graft site will be devoid of any carcinoma or scars with
the use of keratinocyte transplantation. The merits of cultured
keratinocytes have been reviewed and the results obtained
through basic research can be incorporated into the medical
treatment of burns [4]. The anticipated results can be acquired
through donor site healing as well as skin tissue regeneration.
In the light of reality, the appropriate success of healing will be
accomplished by means of cultured keratinocyte transplantation.
It is remarkable to note that, the process of trans-differentiation
of keratinocyte to fibroblast has been proven by using primary
human keratinocytes. Also, similar phenomenon was observed in
the same way while using mouse and rat keratinocytes [5].
Materials and Methods
Reagents
Primary human keratinocytes (PCS-200-010) were purchased
from ATCC collection (ATCC, LGC Standard, and Poland). Human
Primary Keratinocyte cell culture media Cascade BiologicsTM
EpilifeTM (MEPI500CA) and supplemented with Cascade
BiologicsTM Supplement S7(S0175), TrypLE Select, were
purchased from GIBCO (GRAND, Island, NY, USA). Antibody
cytokeratin 5, (CK5) (A2662) and collagen I (A5786) from ABclonal
(ABclonal, USA), Anti-CK10 (ab111447), Fibronectin (ab6328) was
purchased from Abcam (Abcam, Cambridge, UK). Dispase II and
DAPI (4,6-diamidino-2-phenylindole from Sigma Aldrich (Sigma,
USA) [6].
Skin Sample collection & isolation of
Keratinocytes
With the approval of the Institutional Ethics committee, normal
skin biopsies were collected from the extra surgical discards
with written consents from the donor/patients/caregiver.
Samples positive for HIV, HbsAg, HCV, microbial contaminated
skin samples, known cases of cancer, active jaundice, STDs were
excluded from the study. Samples of approximately 2x2 cm
from normal (thigh region) tissue was collected and processed for keratinocyte culture. A maximum of 2x2=4 cm2 tissue was
washed in phosphate-buffered saline (PBS) and incubated in 10
mL of 2.5mgmL (0.25 %), Dispase II in Keratinocyte-specific basal
medium and left for one hour at 37 deg or overnight at 4 °C [7].
Then epidermis was mechanically separated from the dermis with
fine forceps and incubated in TrypLE Select from GIBCO (GRAND,
Island, NY, USA)) for 5-10 min at 37 °C. Upon cellular dissociation,
trypsin activity was reduced by diluting the solution with three
volumes of fresh Human epidermal Keratinocyte-specific medium.
Keratinocytes were then collected through centrifugation and
suspended in Human Primary Keratinocyte cell culture media. The
washing step was repeated 4 to 5 times and finally centrifuged
at 1100 rpm for 5 min. After removing the supernatant, the
cell pellet was re-suspended in a complete keratinocyte culture
medium first with 1 ml. Then total cell number was counted and
finally re-suspended with 10 to 15 ml. Cells were seeded into a 25
cm2 culture flask (BD Falcon, Stockholm, Sweden). Sub-confluent
primary cultures were serially passaged at 1 × 104 cells per cm2.
On every second day, the medium was changed throughout the
study. The primary culture was sub-cultured into 1:3 when subconfluence
had been achieved. It is remarkable to note that, the
isolation methods use serum-free media [8].
Assessment of keratinocyte viability by Trypan
blue Dye
Each time, while passaging, the cell pellets were dissolved in the
1 ml culture medium and from the cell suspension; dilutions were
made in Trypan blue and were taken for counting. The number
of living and dead cells was counted in a haemocytometer, by
triplicate samples of cell suspension 10 μl, each mixed with Trypan
blue 90 μl. Live cell percentage was more than 90 % every time.
Normal Human Keratinocytes were isolated from normal skin
samples obtained from operation theatre and primary cultures
were Initiated and maintained in a replicative state [9].
Trans-differentiation of Primary human
Keratinocytes
The primary human keratinocytes are used, were available
commercially from ATCC (PCS-200-010). The keratinocytes were
seeded in a T-25, Nunc flask for 24 hours. After keratinocyte
morphology appears, media switching is done for dermal
fibroblast. DMEM [GIBCO- REF#10569-010] with 10% fetal
bovine serum [GIBCO- Ref-25030-01] along with L-glutamine
[GIBCO- REF#25030-081] derived the keratinocytes to attain
fibroblast-like morphology. The keratinocyte-specific markers
such as cytokeratin 5 (CK5), PAR2 were used before the start
of transdifferentiation process and after 2 weeks, the dermal
fibroblast specific markers such as collagen -I and fibronectin
were done [10].
Immunocytochemistry
Human primary epidermal keratinocyte cultures were grown on
collagen-coated cover glass. In sub-confluent label fixed in 4%
paraformaldehyde in 1× PBS for 15-20 min and permeabilized in
PBS/ 0.1% Triton X-100 solution for 5 min. Samples were washed
three times with PBS, followed by blocking with 1% BSA (bovine
serum albumin) for 15 min and then incubation with primary antibodies overnight at 4 °C. Dilution of the primary antibody
used as per the datasheet provided by the manufacturer.
Following three washes with PBS, the samples were incubated
with fluorescence-labelled secondary antibodies for 1 h at RT to
visualize the antigens. Additional three washes were given with
PBS and nuclei of the samples were counterstained DAPI and
mounted with Vectashield without DAPI (Life Technologies) and
visualized under a Nikon florescence microscope (Nikon, Japan) [11].
Results
Morphological and growth measurements of
keratinocytes
The use of commercially prepared various kinds of keratinocyte
media such as low-calcium, serum-free medium, the fetal bovine
serum-containing medium was reported by several groups.
However, this method of isolation is used as serum-free media
with high calcium, which is suitable for burn patients. During
the isolation of keratinocytes processes, starting from seeding
the cells to sub-culturing, the number of cells was counted, and
periodical photographs were taken for analysis [12]. The number
of keratinocytes isolated from the total population of cells varied
depending upon the age of the sample. The total number of cells
obtained from 4 sq cm is shown in (Figure 1). About 2.7 million
cells are obtained from 1 square centimetre of skin approximately.
The Keratinocyte became confluent after 7 days and was ready
for sub-culturing. Within a week of time, keratinocytes are
almost 90% confluent. Live image captured on days 1, 3, and 7
as shown in (Figure 2). Sub-culturing, cell counting was done in
each passage number from P1 to P2 and the doubling number
implicates the doubling time of the keratinocytes was found to
be 18-72 hours [13].
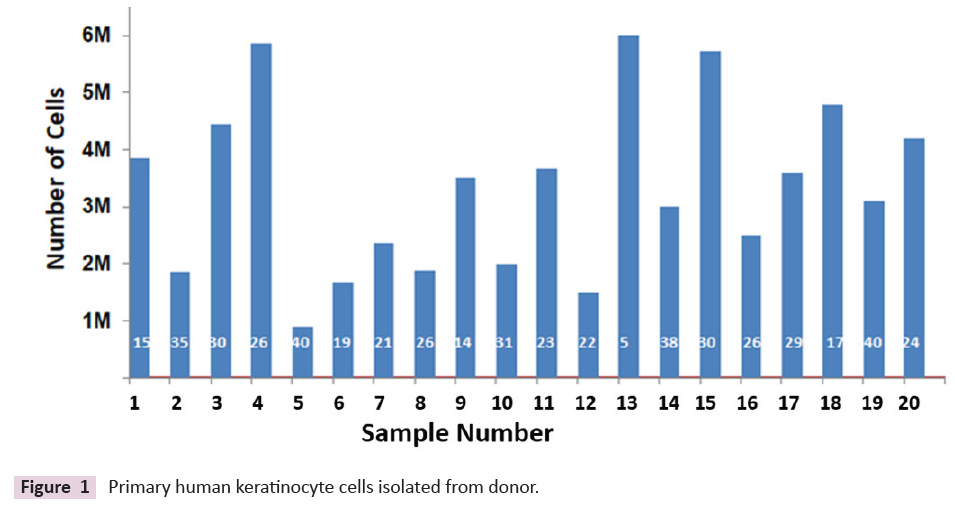
Figure 1: Primary human keratinocyte cells isolated from donor.
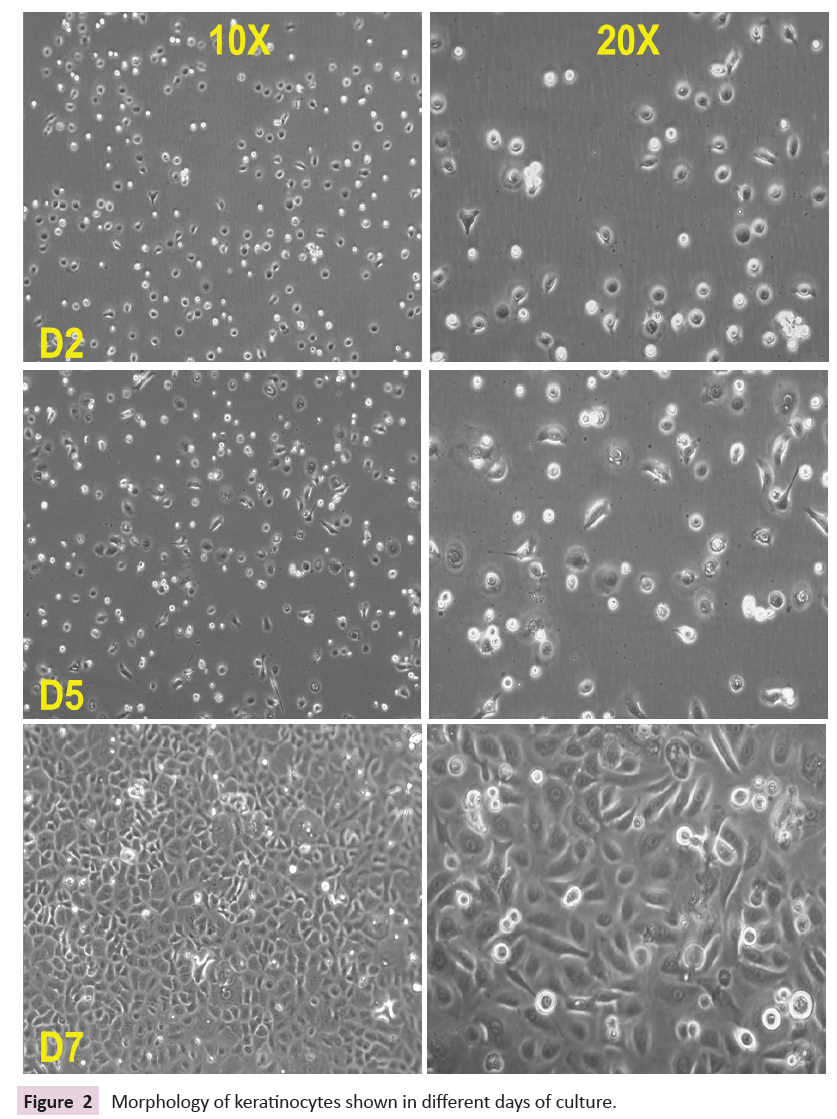
Figure 2: Morphology of keratinocytes shown in different days of culture.
Characterization and Potential of Keratinocytes
While characterizing the keratinocyte, the cytokeratin 5 (CK5) expression pattern has been observed. Among five different age
group samples, cytokeratin 5 expressions have been observed
in the early passage (P1) and it was found that, 86 to 98 % of
expression prominently observed and quantitation, which is
shown in (Figure 3A & 3B). During the subsequent passages,
the percentage of CK5 expression is almost being maintained
up to passage number 7, which indicates that the keratinocyte
population, which was isolated in the proliferative stage, is
retaining the multiplying capacity [14]. It predicts that the
proliferative keratinocyte may bring better wound healing at the
wound site. In addition, the differentiated keratinocyte marker
Cytokeratin 10 (CK10) have been observed in late passages (P6-
P8) and it was found that CK10 expressing cell number increases
from 10-15% only as shown in (Figure 4A & 4B), which clearly
emphasizes that the differentiation capacity would not be
dominated over proliferation. Quantitative analysis of percentage
expression of CK5 and CK10 has been done using image-J software
[15,16].
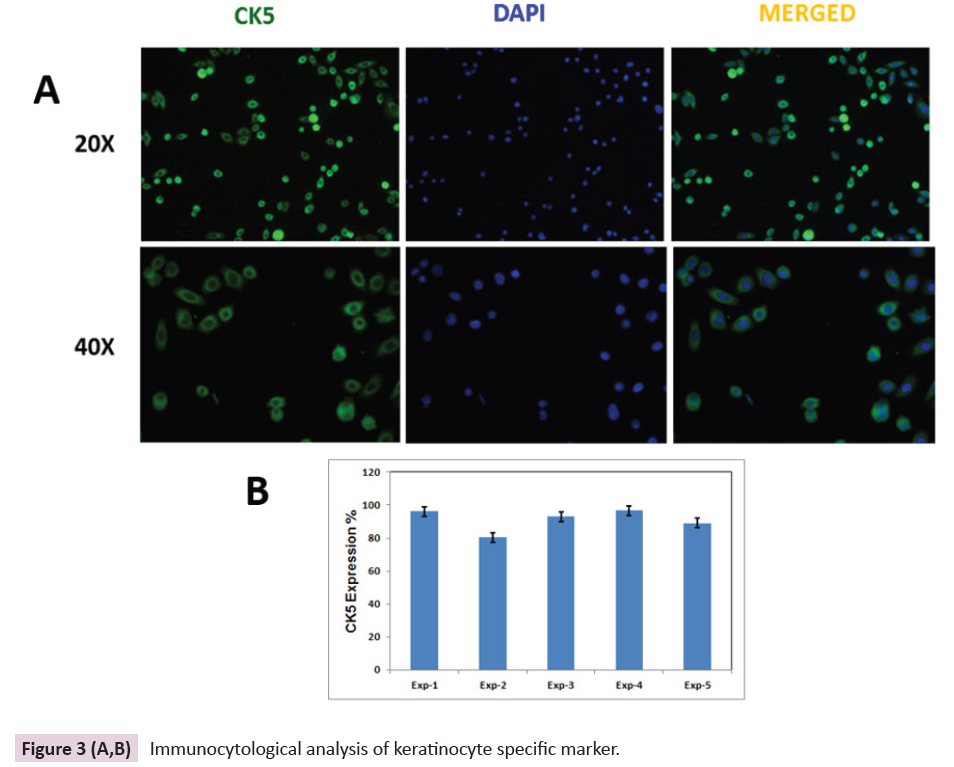
Figure 3 (A,B): Immunocytological analysis of keratinocyte specific marker.
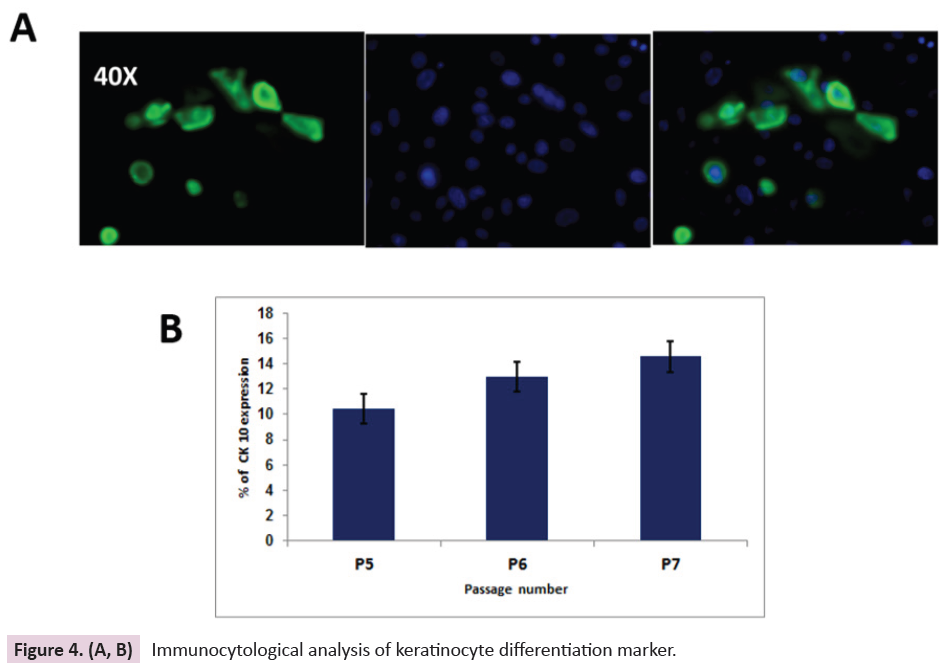
Figure 4. (A, B): Immunocytological analysis of keratinocyte differentiation marker.
Trans-differentiation of Primary Human
Keratinocytes
The primary human keratinocytes, obtained from available
commercial sources followed a similar pattern of proliferation
as primary human keratinocytes isolated directly from human
skin. The doubling time was noted and up to passage no 11 and
it did not change. The expression of CK5 from early passage to
later passage remains almost the same. The collagen I expression
was absent in primary human keratinocytes which is a notable
point. During 2 weeks of trans-differentiation, changes in the
morphology of keratinocytes were observed as fibroblast-like
morphology appeared and bright-field images captured on days
1, 3, 7, and 12 are shown in Figure 5(A, B, C & D). The dermal
fibroblasts play a critical role in synthesizing the extracellular
matrix components (ECM) like collagen I, fibronectin, fibrin which
are considered potent protagonists for the healing of wounds.
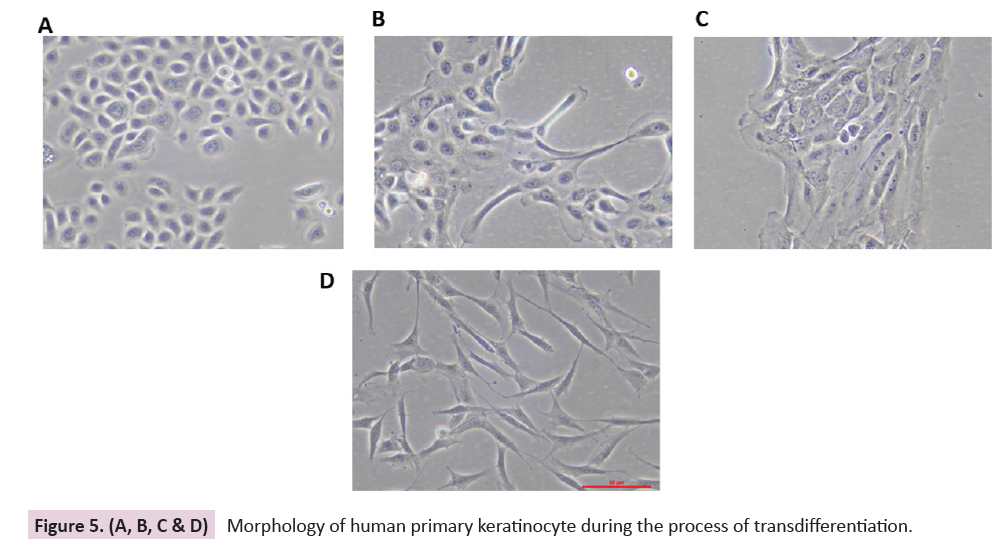
Figure 5. (A, B, C & D): Morphology of human primary keratinocyte during the process of transdifferentiation.
Immunocytological analysis has been done for fibroblast specific
markers and expression of the collagen I and fibronectin and
High magnification of the fibronectin network both are shown in Figure 6(A, B & C).
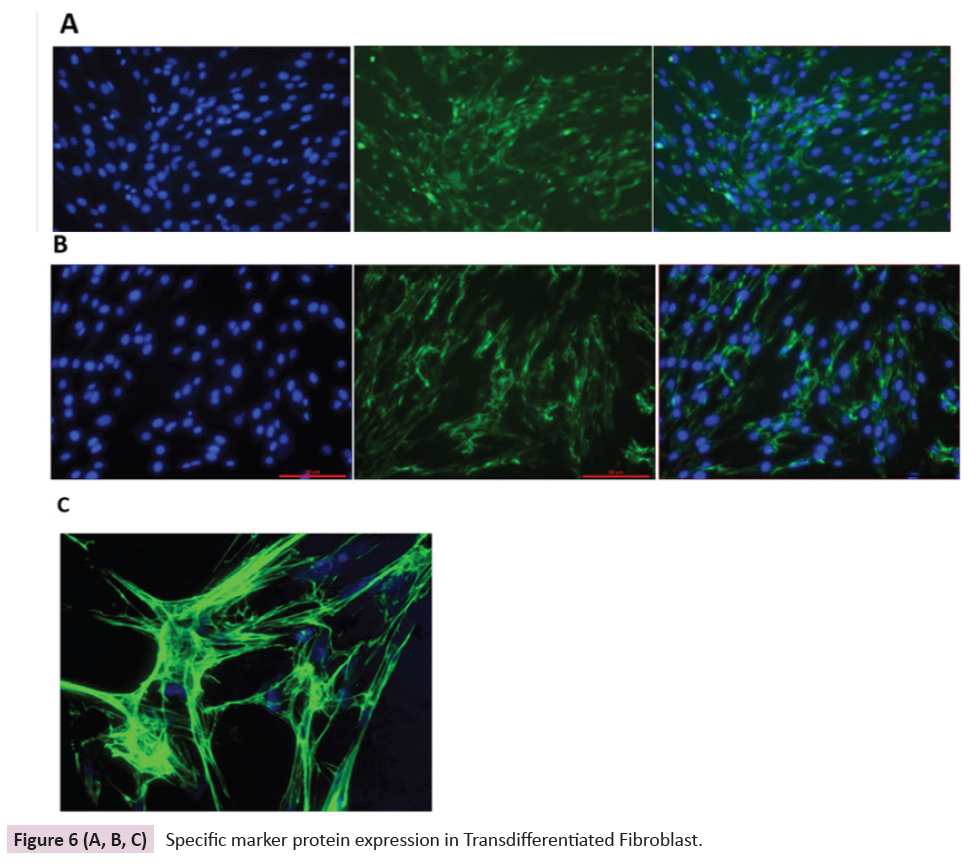
Figure 6 (A, B, C): Specific marker protein expression in Transdifferentiated Fibroblast.
Discussion
Keratinocytes play a vital role in wound closure and are the
most abundant cell type in the epidermis. They prevent
desiccation and provide immunological and barrier mechanisms
of defense against potential pathogens. With the discovery
of the introduction of cultured autologous keratinocytes new
perspective emerged in solving the problem of coverage of extensive burns and diabetic ulcers. The first clinical application
of culture Epithelial Autograft (CEA) was performed in 1981
following the successful serial cultivation of keratinocytes from
skin biopsy by Green et.al in 1979 [17]. But even after three
decades, there are mixed results of the clinical efficacy of the CEA
for the closure of deep burn wounds. But keratinocyte culture
has been proved beneficial in accelerating the wound-healing
when used as an adjunct therapy. Today cultured keratinocytes
are used in many forms (spray, sheets, in composite grafts) for
wound healing around the world. In 1986 madden et.al reported
that allogenic cultured keratinocytes when used in superficial to
deep dermal burns resulted in improved wound healing without any adverse reaction because of non-immunogenic response.
They further confirmed that the allergenic keratinocytes grown
in culture do not express HLA-DR antigen [18]. There are studies
to show that the allergenic keratinocytes secrete the growth
factors required for wound healing. Successful therapeutic use of
allogenic keratinocytes over the last two decades indicates that
it is safe and effective to use on burn wounds and donor sites.
Cultured epithelial autograft (CEA) are aseptically processed
wound dressings, approved by FDA in 2007, and CEA is used for
patients with deep dermal or full-thickness burns greater than
30% TBSA. Hence serum-free media procedure has been used to
culture allogenic keratinocytes which can be used for therapeutic
use [19].
Successful serum-free cultures of the keratinocytes were done
as per the clinical-grade protocol and sterility of the culture has
been maintained throughout different passages and efficacy of
the keratinocytes would be predicted as the cells continued to
be in proliferative stage. Cytokine activation causes keratinocyte
migration in the proliferative phase, leading to closure and
restoration of a vascular network. Keratinocytes can also be
activated by mu-opioid receptor agonists but the role of these
agonists on inflammation and wound closure remains unclear.
Nowadays, several commercialized bioengineered skin products
derived from autologous cells are available. Clinicians harvest
autologous skin, and the company produces a graft-able substrate
seeded with the autologous cells for clinical use in approximately
2 weeks. Here, a non-invasive isolation method was used, with
pure epidermal cells without any dermal contamination and the
isolated keratinocytes continued their proliferative capacity up to
later passages like P11. Immunoreactivity of specific epidermal
cytokeratin used as a marker for proliferation (CK5) has been
confirmed and the differentiation marker (CK10) expression remains under control during the late passages even. Also, there
was no use of feeder layer in the culture system and the capacity
of keratinocytes in retaining the in vivo nature of epidermis.
This evaluation of keratinocyte culture happens within a less
time period (one week) and it facilitates the development of an
enhanced epidermal culture system which would bring hope for
wound healing. Improvement of keratinocyte culture method
in terms of reducing infection risk and elimination of xenobiotic
products indicates the efficacious investigation. In the last two
decades, several views and discussions are going on for use of
keratinocytes in skin tissue engineering, transplantation of
keratinocytes, and making skin substitutes using keratinocytes.
In the future, the use of cultures keratinocytes requires frequent
use of these, and that may, in turn, guarantee the indications of
prescriptions and handing for the successful practice for wound
healing in burns and cutaneous wound healing in diabetic ulcers
[20-28].
Dermal fibroblasts act as an originator, modulator, and which
enable the ECM for the efficient functioning of wound healing. The
trans-differentiation of keratinocyte to fibroblast demonstrates
that keratinocyte stimulates the potential of keratinocyte
to modulate collagen, fibronectin and fibrin synthesis. The
production of soluble growth factors by fibroblast is responsible
for enhancing extracellular matrix deposition. Advances in
cell culture and tissue engineering have made the precise
measurement of extracellular matrix components. Synthesis
of ECM molecules and proteins is done by homing of fibroblast
progenitor cells by migration of keratinocyte progenitor cells
towards the dermal layer as a process of transdifferentiation [29].
Keratinocytes are the executors to accelerate the reepithelialization
process whereby activated keratinocytes
migrate, proliferate, and differentiate to restore the epidermal barrier and keratinocyte transdifferentiated to form dermal
fibroblast to fill the gap for wound healing as shown in model
(Figure 7). Keratinocytes are cells that proliferate rapidly without
losing their capability. Trans-differentiation of keratinocytes
to fibroblast implicates that delivery of healthy cultured
keratinocytes execute as intracellular transmitter system in
the wound microenvironment and consequently heal the
wound effectively. Further trans-differentiation property of
human dermal fibroblast offers a new angle for insight into the
interaction between keratinocytes and fibroblast in the wound
microenvironment [30].
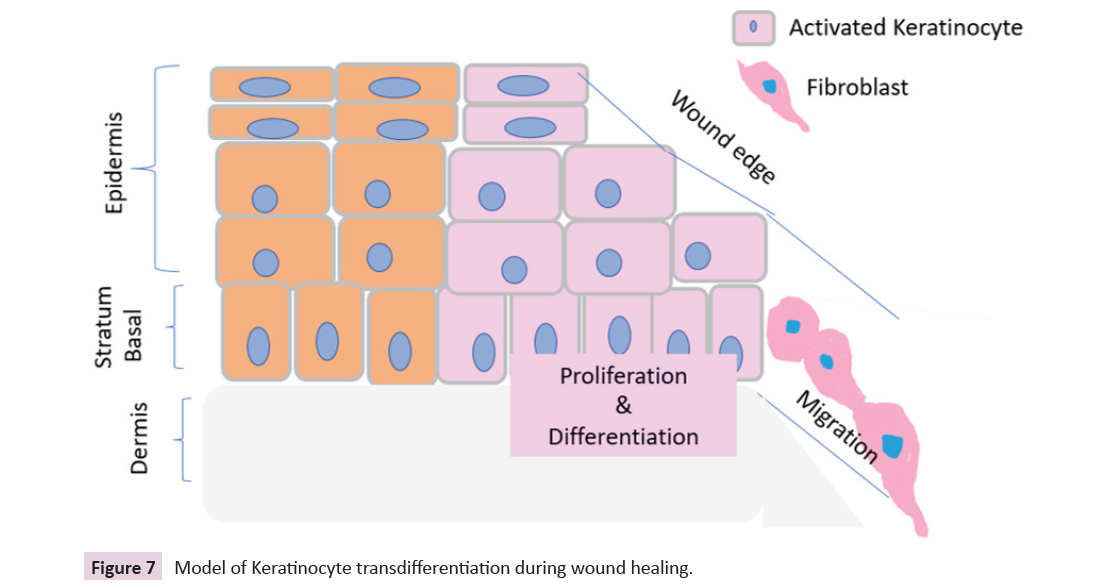
Figure 7: Model of Keratinocyte transdifferentiation during wound healing.
Acknowledgement
The study was supported by the Institutional internal project
grant, National Burn Centre, Airoli, Navi Mumbai, India. The
author gratefully acknowledges the complete support of the
Medical Director, NBC and RCBN skin bank. The author offers
thanks to all the medical and non-medical staff of the National
Burns Centre.
Conflict of Interest
The author has declared no conflict of interest.
References
- Ramezankhani R, Torabi S (2020) Two Decades of Global Progress in Authorized Advanced Therapy Medicinal Products: An Emerging Revolution in Therapeutic Strategies. Front Cell Dev Biol 8:547-653.
Indexed at, Google Scholar, Crossref
- Hwang YG, Lee JW, Park KH, Han SH (2019) allogeneic keratinocyte for intractable chronic diabetic foot ulcers: A prospective observational study. Int Wound J 16:486-491.
Indexed at, Google Scholar, Crossref
- Hosseini Mansoub N (2021) the role of keratinocyte function on the defected diabetic wound healing. Int J Burns Trauma 15:430-441.
Indexed at, Google Scholar
- You HJ, Han SK, Lee JW, Chang H (2012) Treatment of diabetic foot ulcers using cultured allogeneic keratinocytes-a pilot study. Wound Repair Regen 20:491-499.
Indexed at, Google Scholar, Crossref
- Alharbi Z, Piatkowski A, Dembinski R, Reckort S, Grieb G, et al. (2012) Treatment of burns in the first 24 hours: simple and practical guide by answering 10 questions in a step-by-step form. World J Emerg Surg 7:13.
Indexed at, Google Scholar, Crossref
- Herndon DN, Barrow RE, Rutan RL (1989) a comparison of conservative versus early excision: therapies in severely burned patients. Ann Surg 209:547-553.
Indexed at, Google Scholar, Crossref
- Pereira C, Murphy K, Herndon D (2004) Outcome measures in burn care. Is mortality dead? Burns 30:761-771.
Indexed at, Google Scholar, Crossref
- Gallico GG3rd, O’Connor NE, Compton CC (1984) Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med 311:448-451.
Indexed at, Google Scholar, Crossref
- Gallico GG3rd, O’Connor NE (1985) Cultured epithelium as a skin substitute. Clin Plast Surg. 12:149-57.
Indexed at, Google Scholar
- Wood FM, Kolybaba ML, Allen P (2006) the use of cultured epithelial autograft in the treatment of major burn wounds: eleven years of clinical experience. Burns 32:538-544.
Indexed at, Google Scholar, Crossref
- Jackson PC, Hardwicke J, Bamford A, Nightingale P, Wilson Y, et al. (2014) Revised estimates of mortality from the Birmingham Burn Centre, 2001-2010: a continuing analysis over 65 years. Annals of surgery 259:979-984.
Indexed at, Google Scholar, Crossref
- Connor N, Banks-Schlegel S, Kehinde O (1981) Green Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet 1:75-78.
Indexed at, Google Scholar
- Atiyeh BS, Costagliola M (2007) Cultured epithelial autograft (CEA) in burn treatment: three decades later. Burns 33:405-13.
Indexed at, Google Scholar, Crossref
- Munster AM (1996) Cultured skin for massive burns. Ann Surg 224:5-7.
Indexed at, Google Scholar, Crossref
- Auxenfans C, Menet V, Catherine Z, Shipkov H, Lacroix P, et al. (2015) Cultured autologous keratinocytes in the treatment of large and deep burns. Burns. 41:71-9.
Indexed at, Google Scholar, Crossref
- Barret JP, Wolf SE, Desai MH, Herndon DN (2000) Cost-efficacy of cultured epidermal autografts in massive pediatric burns. Ann Surg 231:869-876.
Indexed at, Google Scholar, Crossref
- Auxenfans C, Menet V, Catherine Z, Shipkov H, Lacroix P, et al. (2015) Cultured autologous keratinocytes in the treatment of large and deep burns: a retrospective study over 15 years. Burns. 41:71-79.
Indexed at, Google Scholar, Crossref
- Auxenfans C, Shipkov H, Bach C, Catherine Z, Lacroix P, et al. (2014) Cultured allogenic keratinocytes for extensive burns: a retrospective study over 15 years. Burns 40:82-8.
Indexed at, Google Scholar, Crossref
- Sakamoto M, Ogino S, Shimizu Y, Inoie M, Lee S, et al. (2020) Human cultured epidermis accelerates wound healing regardless of its viability in a diabetic mouse model. PLoS One 15:237-985.
Indexed at, Google Scholar, Crossref
- Sakamoto M, Morimoto N, Inoie M, Takahagi M, Ogino S, et al. (2017) Cultured Human Epidermis Combined With Meshed Skin Autografts Accelerates Epithelialization and Granulation Tissue Formation in a Rat Model. Ann Plast Surg 78:651-658.
Indexed at, Google Scholar, Crossref
- Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, et al. (2014) Epithelialization in wound healing: a comprehensive review. Adv Wound Care 3:445-464.
Indexed at, Google Scholar, Crossref
- Leong C, Neumann C, Ramasamy S, Rout B, Yi Wee L, et al. (2017) Investigating endogenous µ-opioid receptors in human keratinocytes as pharmacological targets using novel fluorescent ligand. PLoS 12:18-27.
Indexed at, Google Scholar, Crossref
- Bigliardi PL, Buchner S, Rufli T, Bigliardi-Qi M (2002) Specific stimulation of migration of human keratinocytes by mu-opiate receptor agonists. J Recept Signal Transduct Res 22:19-29.
Indexed at, Google Scholar, Crossref
- Lootens L, Brusselaers N, Beele H, Monstrey S (2013) Keratinocytes in the treatment of severe burn injury: an update. Int Wound J 10:6-12.
Indexed at, Google Scholar, Crossref
- Metcalfe AD, Ferguson MW (2007) Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface 4:413-437.
Indexed at, Google Scholar, Crossref
- Gardien KL, Middelkoop E, Ulrich MM (2014) Progress towards cell-based burn wound treatments. Regen Med 9:201-18.
Indexed at, Google Scholar, Crossref
- Chua AWC, Khoo YC, Tan BK, Tan KC, Foo CL, et al. (2016) Skin tissue engineering advances in severe burns: review and therapeutic applications. Burns & Trauma 4:1-14.
Indexed at, Google Scholar, Crossref
- Butler KL, Goverman J, Ma H, Fischman A, Yu YM, et al. (2010) Stem cells and burns: review and therapeutic implications. Journal of burn care & research 20:10-31.
Indexed at, Google Scholar, Crossref
- Tracy LE, Minasian RA, Caterson EJ (2016) Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv Wound Care (New Rochelle) 5:119-136.
Indexed at, Google Scholar, Crossref
- Wang HJ, Pieper J, Schotel R, Blitterswijk CA, Lamme EN, et al. (2004) Stimulation of skin repair is dependent on fibroblast source and presence of extracellular matrix. Tissue Eng 10:1054-1064.
Indexed at, Google Scholar, Crossref
Citation: Mishra M (2023) The TransDifferentiation of Keratinocyte: Requisite for
Skin Wound Healing and Cosmetic Surgery.
Int J Drug Dev Res J, Vol. 15 No. 2: 997.













