Keywords
Pulmonary Tuberculosis; Rifampicin; Associated risk factors; Trend; Ethiopia
Abbreviations: HIV: Human Immunodeficiency Virus; M. tuberculosis: Mycobacterium Tuberculosis; MDR-TB: Multidrug Resistance Tuberculosis; RIF: Rifampicin; TB: Tuberculosis; WHO: World Health Organization
Introduction
Tuberculosis is still the problematic and kills large number of people globally [1,2]. Despite the availability of chemotherapy, the catastrophic effect of HIV on TB in Africa got global concern to improve TB prevention and control [3]. One-third of world population is currently infected with TB mainly with latent infection and around 8 million people develop TB disease in every year and from these nearly 2 million death result and Ethiopia is one top Tb burden country in Africa [4]. From 30 countries with high MDR-TB globally, Ethiopia was one of them with estimated 5800 cases in 2016 [5]. Low achievement of global Tb control was due to slow, insensitive diagnostic methods, particularly for the detection of drug-resistant forms and in patients with human immunodeficiency virus infection. Early detection is essential to reduce the death rate and interrupt transmission [6]. Current and future control of TB relies on the correct use of new diagnostics and new drugs from one side, and on the consistent application of the five core interventions at the programmatic level. In addition, it is mandatory to tackle the social determinants and socio-economic barriers favoring the MDR-TB, otherwise it will not be possible to reach the planned goals as well as TB Elimination [7]. Ethiopia implemented different advanced technologies concordantly with WHO recommendations, including the use of the GeneXpert™ MTB/ RIF assay to achieve the global strategy on TB [8]. Different studies conducted at hospital level and research institutions yielded different results on the trends of TB and rifampicin resistance in Ethiopia [9-12], and most of those were done at hospital level, no researches were done on trends of TB and rifampicin resistance at health center. Globally, almost 0.5 million cases of multidrugresistant TB (MDR-TB) with bacillary resistance to at least isoniazid and rifampicin are estimated to emerge every year. Rifampicin is one of the most potent first-line anti-TB drugs. It inhibits the transcription and elongation of RNA by binding to the b-subunit of RNA polymerase [13]. Detection of rifampicin resistance in Mycobacterium tuberculosis isolates use as an initial indicator of multidrug resistance [14]. Even though, Ethiopia is one of the high burden countries, regarding both in TB incidence and MDR-TB [15] there is limited information regarding the trend analysis of mycobacterium tuberculosis and rifampicin resistance in southwestern part of Ethiopia particularly in study area. To the best of our knowledge, there are no studies conducted to identify the trends in Mycobacterium tuberculosis and rifampicin resistance using GeneXpert™ in south-west. Therefore, this study aimed to determine the trends in pulmonary tuberculosis and rifampicin resistance using GeneXpert™ among TB-presumptive cases at Wolkite health center, Southern Ethiopia.
Methods and Materials
Study design, area and period
The study was conducted in Wolkite Town located 158 km southwest of Addis Ababa along the Jimma Road in the Southern Region of Ethiopia. Wolkite town, the capital of Gurage Zone, has a latitude and longitude of 8°17'N37°47'E and an elevation between 1910 and 1935 m above sea level. Based on the 2007 census conducted by the central statistical agency shows a total population of 28,866 of whom 15,704 are and 13,792 women. Institution- based retrospective study design was used to gather existing data from TB patients’ registration logbook at Wolkite Town Health Center. Wolkite town Health center is serving the people in wolkite town and catchment areas including provision TB diagnosis using GeneXpert™ and treatment.
Eligibility
Inclusion and exclusion criteria criteria: Participants with complete data in the GeneXpert™ TB registration book were included during the study period of last 5 years (2016-2020) and those cases with indeterminate and/or invalid results were excluded from the study.
Independent variables: patients age, sex, residence, year of diagnosis, reason of diagnosis and HIV status.
Dependent Variable: Mycobacterium Tuberculosis and Rifampicin (resistance or sensitivity).
Sample size, data collection and analysis
The secondary data was collected retrospectively for all presumptive TB patients from a GeneXpert™ TB registration book from February 2016, to January 2020. Laboratory based and Sociodemographic data were extracted from registration logbook by structured questionnaire and checklist by reviewing five years TB results from Wolkite Town Health Center Laboratory. Then, data were recorded from patient’s case logbook in well-prepared checklist format containing dependent (outcome) variable and independent variables. After checking its completeness and consistency of data to maintain its quality, it was entered in SPSS version 21 and analyzed. The descriptive data was presented using tables and graphs/figures.
Data analysis and interpretation
Data obtained through the checklist and laboratory test results were entered to SPSS 21 for statistical analysis. Descriptive analysis, frequencies, and figures were used to explain the findings. Bivariate Logistic regression analysis was conducted primarily to check the association of each independent variable with the dependent variable at P value <0.2. Multivariate logistic regression models were employed to analyze specific associations between variables. Odds ratio (OR) and 95% confidence interval (CIs) were calculated using logistic regression model to measure the strength of an association. In all cases, p-values less than 0.05 were considered statistically significant.
Ethical consideration
The study was cleared ethically by college of health sciences and medicine, institutional research ethical committee, Wolkite University. Then formal letter of cooperation was written to Wolkite town health center administration. The objective of the study was explained to the health center. The letter of agreement was attached to ensure confidentiality of data. The information taken from patients’ recorded data were kept confidential. Only codes were used to identify the study groups.
Result
Socio-demographic and clinical characteristics
From a total of 3504 TB clinically suspected patients that have submitted their samples for TB diagnosis in five year, 3286 have complete data in the registration log books. Of the total of study participants, majority were males 1787 (54.4%), majority were found in age group of 35-44 734 (22.3%) followed by 25-34 and 45-55 which both accounted 643 (19.6%). Most of the reason for TB diagnosis was presumptive TB case 2669 (81.2%), while presumptive drug resistant TB reason was 580(17.7%). Among the presumptive TB patients’ took diagnosis, majority were new cases 2898 (88.2%). The number of diagnosis of Mycobacterium tuberculosis using the GeneXpert™ have increased between 2016 and 2018 and decreased between 2019 and 2020, whereas between 2018 and 2019, there was no significant change of TB diagnosis. From the total of study participants only 560 (17.04%) know their HIV status, from which 143 (25.5%) were living with HIV, that is TB/HIV co-infection (Table 1).
| Variables |
Frequency |
Percent (%) |
| Year of diagnosis |
| 2016 |
147 |
4.5 |
| 2017 |
597 |
18.5 |
| 2018 |
967 |
29.4 |
| 2019 |
945 |
28.8 |
| 2020 |
630 |
19.2 |
| Sex |
| Male |
1787 |
54.4 |
| Female |
1479 |
45.0 |
| Unknown |
20 |
0.6 |
| Age |
| 0-4 |
50 |
1.5 |
| 5-14 |
246 |
7.5 |
| 15-24 |
431 |
13.1 |
| 25-34 |
643 |
19.6 |
| 35-44 |
734 |
22.3 |
| 45-54 |
643 |
19.6 |
| ≥ 55 |
505 |
15.4 |
| Unknown |
34 |
1.0 |
| Residence |
| Urban |
1503 |
45.7 |
| Rural |
1588 |
48.3 |
| Unknown |
195 |
6 |
| Reason of diagnosis |
| Presumptive TB |
2669 |
81.2 |
| Presumptive DR Tb |
580 |
17.7 |
| Unknown |
37 |
1.1 |
| TB registration type |
| New |
2898 |
88.2 |
| Failure |
5 |
0.2 |
| Relapse |
170 |
5.2 |
| Defaulter |
3 |
0.1 |
| Unknown |
210 |
6.4 |
| HIV status |
| Reactive |
143 |
4.4 |
| Non-reactive |
417 |
12.7 |
| Unknown |
2726 |
83.0 |
Table 1 Socio-demographic and clinical characterstics Presumptive TB patients in Wolkite town health center, 2020.
Prevalence, trend of mycobacterium tuberculosis and rifampicin resistance
The prevalence of all forms of TB detection was 27.4% (899/3286) in five years of the study. From total positive TB, refampicin resistance identified among 137 (15.2%). Mycobacterium tuberculosis was reported in all age groups, but the infection rate was higher in the age groups 25-34 years, with a prevalence rate of 209 (23.2%) followed by 45- 55 age group with a prevalence rate of 176 (19.6%). From the total confirmed all forms of Mycobacterium tuberculosis cases, 13.5% (121/899) were identified from presumptive TB and 1.8% (16/899) from presumptive drug resistance-TB. Of the total of confirmed positive cases, 14.6% (121/826) and 23.5% (16/68) were rifampicin resistance of presumptive tuberculosis and presumptive drug resistance tuberculosis patients respectively.
The number patients visited to take TB diagnosis increased from 2016 to 2018, whereas it was decreased from 2018 to 2020 as show Figure 1.
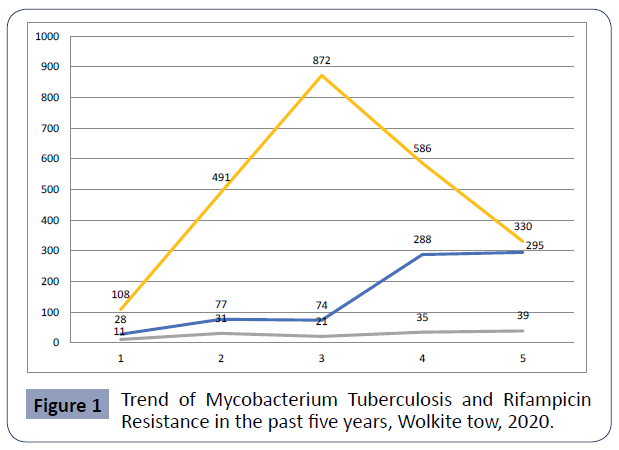
Figure 1 Trend of Mycobacterium Tuberculosis and Rifampicin Resistance in the past five years, Wolkite tow, 2020.
Discussion
In this study, five-year retrospective data collected to determine the trend TB among clientrs visited to TB clinic of wolkite town heath center. The overall detection rate of TB in study was 27.4%. This finding was comparable with study done in Eastern zone of Tigrai, 24.3% [9] Debre Markos Hospital 23.2% [16], Gambella 20.0% [17] Gondar Referral Hospital (24.6%) [18], Afar (24.5%) [19], South Africa 26% [20], Nigeria 22.9% [21], Southern Sierra Leone 21.3% [22], and report by WHO in Africa 25% [23]. However, our study showed higher overall Mycobacterium tuberculosis prevalence than other studies conducted in Eastern Ethiopia 15.7% [24], Northwestern Tigrai, Ethiopia 9.9% [25], Debre Berhan, Ethiopia 13% [26], Felege Hiwot Referral Hospital and Debre Tabor Hospitals 14.6% [27], Addis Ababa 15.11% [28], Nigeria 10.3% [29]. In contrary, our study revealed lower prevalence of mycobacterium tuberculosis compared to other stsudies conducted in Jigjiga, Ethiopia 65.5% [30], Kenya 32.2% [31], and Congo 79.1% [23]. This difference might be due difference in the method of diagnosis, duration of study period, community characteristics and geographical location, skill of the laboratory personnel to detect TB, study design and other factors that may affect case occurrences in different study areas. The rate of rifampicin resistance was 15.2% in this study among confirmed TB cases. This result was agreed with studies done in Debre Markos Referral Hospital 10.3% [32], Felege Hiwot Referral Hospital and Debre Tabor Hospital 9.3% [27], Addis Ababa 9.9% [28] and India 10.5% [33] and Nepal 10.2% [34]. However, this finding was higher than the study done in Eastern zone of Tigrai, Ethiopia 9.1% [9], Eastern Ethiopia 4.1% [24], Northwestern Ethiopia 8.7% [25], Debre Berhan, Ethiopia 5.2% [26]. But higher rifampicin resistance rate was reported in this study than studies done in selected hospitals Addis Ababa 7.8% [28], Nigeria 2.9% [29]. The possible reason for variations could be related to differences in study designs and drug utilization manner of patients. Regarding the age group, 25-34 years were more affected by MTB 209 (23.2%) in the current study followed by 45-55 years 176(19.6%). This was consistent with previous reports in different part of Ethiopia cited elsewhere above. This might be due to more exposure to the outer environment, high workload and wide range of mobility of young people to acquire the TB bacilli.
In the current study, the Mycobacterium tuberculosis detection rate was higher in presumptive TB patients (30.9%) compared to presumptive drug resistance TB patients (11.7%). Our study was consistent with other studies Gondar, Ethiopia 25.2% [18] and Zimbabwe 37.1% [35]. In opposite, this finding showed higher prevalence among Presumptive TB patients than studies conducted in Afar 20.9%(19) Debre Markos Referral Hospital 15.1% [36] and Gambella 19.6% [17]. However, it is much lower than studies conducted in Felege Hiwot Referral Hospital and Debre Tabor Hospital 54.8% [17], The discrepancies might be due to included TB cases, number of study participants.
In this study, sex, age, residence, reason for diagnosis, patient registration group, previous history of TB, HIV status of patients were found to be significantly associated with the disease caused by mycobacterium (p<0.005). In general, most of study participants were new cases as supported many researches referenced in this study. HIV-TB co-infection was identified among 28.8% (41/142) in this study showing for majority of study participants, the HIV status was unknown as shown in Table 2.
| Variables |
M. tuberculosis result by GeneXpert™ |
COR (95%CI) |
p-value |
| |
Detected |
Not detected |
|
|
| Sex |
P=0.375 |
| Male |
500 |
1287 |
1 |
| Female |
394 |
1085 |
1.071(0.921,1.244) |
| not known |
5 |
15 |
|
| Age |
p>0.05 |
| 0-4 |
13 |
37 |
.876 (.318,2.413) |
| 5-14 |
67 |
179 |
.822 (.355, 1.905) |
| 15-24 |
143 |
288 |
.620 (.274,1.403) |
| 25-34 |
209 |
434 |
.639 (.284,1.435) |
| 35-44 |
170 |
564 |
1.021 (.454, 2.296) |
| 45-54 |
176 |
467 |
.816 (.363, 1.837) |
| ≥ 55 |
113 |
392 |
1.067 (.470, 2.423) |
| Unknown |
8 |
26 |
1 |
| Residence |
P=0.003 |
| Urban |
384 |
1119 |
1 |
| Rural |
443 |
1145 |
.825 (.725, .935) |
| Unknown |
72 |
123 |
x |
| Reason of diagnosis |
P=0.03 |
| Presumptive TB |
826 |
1842 |
.867 (.762, .986) |
| Presumptive DR TB |
68 |
513 |
2.696 (2.325, 3.793) |
| Unknown |
5 |
32 |
1 |
| TB registration type |
P=0.000 |
| New |
660 |
2238 |
1.453 (1.068, 1.977) |
| Failure |
5 |
0 |
X |
| Relapse |
168 |
2 |
0.005 (.001, .21) |
| Defaulter |
3 |
0 |
|
| Unknown |
63 |
147 |
1 |
| Year of diagnosis |
P<0.01 |
| 2016 |
38 |
109 |
2,658 (1.780, 3.969) |
| 2017 |
106 |
491 |
4.292 (3.304, 5.576) |
| 2018 |
94 |
873 |
8.606 (6.609, 11.206) |
| 2019 |
358 |
587 |
1.519 (1.239, 1.864) |
| 2020 |
303 |
327 |
1 |
| HIV status |
P<0.01 |
| Reactive |
41 |
101 |
|
| Non-reactive |
169 |
248 |
1.402 (1.216, 1.610) |
| Unknown |
688 |
2038 |
1 |
Table 2 the prevalence of mycobacterium tuberculosis Among Presumptive Clients in wolkite town, Ethiopia, 2020.
Conclusion and Recommendation
In this study, the overall trends of Mycobacterium tuberculosis and rifampicin resistance were found to be significant and with variation each year. Rifampicin resistance is more common in presumptive drug resistance tuberculosis individuals. Therefore, maximizing early detection of drug-resistant and strengthening tuberculosis infection control activities are recommended to reduce the burden of this contagious and potentially deadly disease.
Limitation of the study
As retrospective data was collected from the GeneXpert™ TB registration book, missing and incompleteness of data was faced.
Acknowledgments
We would like to thank Wolkite University College of Health Science and Medicine Research Ethical Committee for approval to conduct this research and wolkite health center laboratory for documented information about GeneXpert™. We also wish to express our sincere thanks and appreciation to all Wolkite town Health center laboratory staff for their supporting and communication in accessing necessary documents.
Authors’ Contributions
AH: Conceived the study, participated in the study design and data analysis, drafted and finalized the manuscript for publication. KH: Participated in the data collection, data processing, and data analysis. SS: Participated in the study design, data acquisition, and data analysis. All authors contributed to the writing of the paper. All authors read and approved the final manuscript.
Funding
The research was funded by Wolkite University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
First ethical clearance was obtained from the Wolkite University ethical review board. Written informed consent was obtained from Health center and any information related to the participants was kept confidential and coded without mentioning their names.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
40396
References
- Netikul T, Palittapongarnpim P, Thawornwattana Y, Plitphonganphim S (2021) Estimation of the global burden of Mycobacterium tuberculosis lineage 1. Infect Genet Evol 91: 104802.
- Ragonnet R, Trauer JM, Geard N, Scott N, McBryde ES (2019) Profiling Mycobacterium tuberculosis transmission and the resulting disease burden in the five highest tuberculosis burden countries. BMC Med 17: 1-12.
- Glaziou P, Sismanidis C, Floyd K, Raviglione M (2015) Global epidemiology of tuberculosis. Cold Spring Harb Perspect Med 5: a017798.
- Corbett EL, Bandason T, Cheung YB, Munyati S, Godfrey-Faussett P, Hayes R, et al. (2007) Epidemiology of tuberculosis in a high HIV prevalence population provided with enhanced diagnosis of symptomatic disease. PLoS Med 4: e22.
- Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, et al. (2010) Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363: 1005-1015.
- Matteelli A, Centis R, D’Ambrosio L, Sotgiu G, Tadolini M, et al. (2016) WHO strategies for the programmatic management of drug-resistant tuberculosis. Expert Rev Respir Med 10: 991-1002.
- FMOH (2014) Guidelines on Programmatic Management of Drug Resistant Tuberculosis in Ethiopia. Federal Democratic Republic of Ethiopia Ministry of Health, Ethiopia.
- Abay GK, Abraha BH (2020) Trends of Mycobacterium tuberculosis and rifampicin resistance in Adigrat General Hospital, Eastern zone of Tigrai, North Ethiopia. Trop Dis Travel Med Vaccines 6: 1-9.
- Asebe G, Ameni G, Tafess K (2014) Ten years tuberculosis trend in Gambella regional hospital, Southwestern Ethiopia. Malays J Med Biol Res 1: 18-24.
- Alemu T, Gutema H (2019) Trend in magnitude of tuberculosis in Awi Zone, Northwest Ethiopia: a five-year tuberculosis surveillance data analysis. BMC Res Notes 12: 1-5.
- Gashu Z, Jerene D, Datiko D, Hiruy N, Negash S, et al. (2018) Seasonal patterns of tuberculosis case notification in the tropics of Africa: a six-year trend analysis in Ethiopia. PloS One 13: e0207552.
- Siu GKH, Zhang Y, Lau TC, Lau RW, Ho PL, et al. (2011) Mutations outside the rifampicin resistance-determining region associated with rifampicin resistance in Mycobacterium tuberculosis. J Antimicrob Chemother 66: 730-3.
- Traore H, Fissette K, Bastian I, Devleeschouwer M, Portaels F (2000) Detection of rifampicin resistance in Mycobacterium tuberculosis isolates from diverse countries by a commercial line probe assay as an initial indicator of multidrug resistance. Int J Tuberc Lung Dis 4: 481-484.
- Girum T, Muktar E, Lentiro K, Wondiye H, Shewangizaw M (2018) Epidemiology of multidrug-resistant tuberculosis (MDR-TB) in Ethiopia: a systematic review and meta-analysis of the prevalence, determinants and treatment outcome. Trop Dis Travel Med Vaccines 4: 1-12.
- Mulu W, Abera B, Yimer M, Hailu T, Ayele H, et al. (2017) Rifampicin-resistance pattern of Mycobacterium tuberculosis and associated factors among presumptive tuberculosis patients referred to Debre Markos Referral Hospital, Ethiopia: a cross-sectional study. BMC Res Notes 10: 1-8.
- Ejeta E, Beyene G, Bonsa Z, Abebe G (2018) Xpert MTB/RIF assay for the diagnosis of Mycobacterium tuberculosis and Rifampicin resistance in high human immunodeficiency virus setting in gambella regional state, southwest ethiopia. J Clin Tuberc Other Mycobact Dis 12: 14-20.
- Jaleta KN, Gizachew M, Gelaw B, Tesfa H, Getaneh A, et al. (2017) Rifampicin-resistant Mycobacterium tuberculosis among tuberculosis-presumptive cases at University of Gondar Hospital, northwest Ethiopia. Infect Drug Resist 10: 185.
- Gebrehiwet GB, Kahsay AG, Welekidan LN, Hagos AK, Abay GK, et al. (2019) Rifampicin resistant tuberculosis in presumptive pulmonary tuberculosis cases in Dubti Hospital, Afar, Ethiopia. J Infect Dev Ctries 13: 21-27.
- Stevens WS, Scott L, Noble L, Gous N, Dheda K (2017) Impact of the GeneXpert MTB/RIF technology on tuberculosis control. Microbiol Spectr 5: 17.
- Ikuabe PO, Ebuenyi ID (2018) Prevalence of rifampicin resistance by automated Genexpert rifampicin assay in patients with pulmonary tuberculosis in Yenagoa, Nigeria. Pan Afr Med J 29.
- Babawo L, Sellu E, George A, Kaikai D (2020) A Five-Year Incidence Trend Analysis of Tuberculosis (TB) and HIV/AIDs Co-Infection at Bo Government Hospital, Southern Sierra Leone. J Public Health Dis Prev 3: 2.
- Farra A, Manirakiza A, Yambiyo BM, Zandanga G, Lokoti B, et al. (2019) Surveillance of rifampicin resistance with GeneXpert MTB/RIF in the National Reference Laboratory for tuberculosis at the Institut Pasteur in Bangui, 2015–2017. Open Forum Infect Dis 6: ofz075.
- Bodena D, Ataro Z, Tesfa T (2019) Trend analysis and seasonality of tuberculosis among patients at the Hiwot Fana Specialized University Hospital, Eastern Ethiopia: a Retrospective Study. Risk Manag Healthc Policy 12: 297.
- Negash H, Legese H, Adhanom G, Mardu F, Tesfay K, et al. (2020) Six Years Trend Analysis of Tuberculosis in Northwestern Tigrai, Ethiopia; 2019: A Retrospective Study. Infect Drug Resist 13: 643.
- Asfaw T, Terefe A, Nigus M (2018) Patterns and Trends of Rifampicin-Resistance Mycobacterium tuberculosis and Associated Factors among Presumptive Tuberculosis Patients at Debre Berhan Referral Hospital. J Bioprocess Biotech 8: 2.
- Derbie A, Worku S, Mekonnen D, Mezgebu Y, Teshager A, et al. (2016) Xpert MTB/RIF assay for the diagnosis of Mycobacterium tuberculosis and its Rifampicin resistance at Felege Hiwot and Debre Tabor Hospitals, Northwest Ethiopia: A preliminary implementation research. Ethiop J Health Dev 30: 60-66.
- Arega B, Menbere F, Getachew Y (2019) Prevalence of rifampicin resistant Mycobacterium tuberculosis among presumptive tuberculosis patients in selected governmental hospitals in Addis Ababa, Ethiopia. BMC Infect Dis 19: 1-5.
- Azuonwu O, Ihua N, Kpomasiruchi W (2017) Molecular detection of Mycobacterium tuberculosis (MTB) and rifampicin resistant strain among subjects accessing health care at federal medical centre, Yenegoa, Bayelsa State; Nigeria. Yenegoa, Bayelsa state. Health Sci J 8: 120.
- Worku M, Agonafir M, Yassin MA, Yassin MA, Datiko DG, et al. (2019) Use of Xpert MTB/RIF for the Identification of TB and Drug Resistance Among Smear-Negative and Re-Treatment Cases in Rural Areas of Ethiopia. Open Microbiol J 13.
- Muia P, Ngugi M, Mburu D (2017) Performance of GeneXpert assay in detecting pulmonary tuberculosis and Rifampicin resistance in patients attending Kitui County hospital, Kenya. J Trop Dis 5: 246.
- Eshetie S, Gizachew M, Alebel A, van Soolingen D (2018) Tuberculosis treatment outcomes in Ethiopia from 2003 to 2016, and impact of HIV co-infection and prior drug exposure: A systematic review and meta-analysis. PLoS One 13: e0194675.
- Gupta A, Mathuria JP, Singh SK, Gulati AK, Anupurba S (2011) Antitubercular drug resistance in four healthcare facilities in North India. J Health Popul Nutr 29: 583-592.
- Sah SK, Bhattarai PR, Shrestha A, Dhami D (2020) Rifampicin-resistant Mycobacterium tuberculosis by GeneXpert MTB/RIF and associated factors among presumptive pulmonary tuberculosis patients in Nepal. Infect Drug Resist 13: 2911-2919.
- Makamure B, Mhaka J, Makumbirofa S, Mutetwa R, Mupfumi L, et al. (2013) Microscopic-observation drug-susceptibility assay for the diagnosis of drug-resistant tuberculosis in Harare, Zimbabwe. PloS One 8: e55872.
- Kelecha WT, Teklegiorgis SGS, Gemechu MM (2021) Rifampicin-resistance Pattern of Mycobacterium Tuberculosis and Associated Risk Factors Among Presumptive Pulmonary and Extra Pulmonary Tuberculosis Patients at Madda Walabu University Goba Referral Hospital, Southeast Ethiopia. Research Square Blog.






