Review Article - (2022) Volume 0, Issue 0
Use of Anti-Mononuclear Antibodies as Therapy for Covid-19
Jhon Fredy Bello Cordero1*,
Urbano David Benitez Russo2,
Fernan Andres Torres Hernandez3,
Jesus Miguel Moreno Martinez4,
Mayerly del Carmen Mercado Oliva5,
Daniel Nicolas Pinilla Lopez6,
Miguel Angel Iglesias Herrera7 and
Jhoyner Alberto Jimenez Filigrana8
1Medical Specialist Emergency Medicine, Fundacion Universitaria de Ciencias de la Salud Bogota, Colombia
2Internist, Universidad Centro Occidental Lisando Alvarado, Venezuela
3General Physician, Universidad de Cartagena, Colombia
4General Physician, Universidad Del Sinu, Monteria, Colombia
5General Physician, Universidad de Sucre, Colombia
6General Physician, Universidad del Rosario, Bogota, Colombia
7General Physician, Universidad del Sinu, Cartagena, Colombia
8General Physician, Universidad El Bosque, Bogota, Colombia
*Correspondence:
Jhon Fredy Bello Cordero, Medical Specialist Emergency Medicine, Fundacion Universitaria de Ciencias de la Salud Bogota,
Colombia,
Email:
Received: 22-Apr-2022, Manuscript No. Iphsj-22-12747;
Editor assigned: 24-Apr-2022, Pre QC No. PreQC No. Iphsj-22-12747 (PQ);
Reviewed: 27-May-2022, QC No. QC No. Iphsj-22-12747;
Revised: 01-Jun-2022, Manuscript No. Iphsj-22-12747(R);
Published:
09-Jun-2022, DOI: 10.36648/1791-809X.16.S7.948
Abstract
The disease caused by a B-coronavirus, an RNA virus, had its beginnings in 2019 in Wuhan - China where a series of cases of pneumonia caused by this agent were identified, after sequencing the nucleic acid of the epithelial cells of the lower respiratory tract of 4 patients with confirmed pneumonia of unknown cause by real�time reverse transcription PCR at Beijing Hospital, a new beta coronavirus 2019-nCoV, which was later named SARS-CoV-2, was found. Currently there are only maintenance therapies and symptom management, since there are no exact therapies for the management of severe SarsCoV2 infection, antiviral drugs and other drugs used to manage the infection such as acyclovir, panciclovir, chloroquine are used , ganciclovir, remdesivir, nitazoxadine, corticosteroids among other medications currently used for symptom management- The use of neutralizing antibody therapy may alter the course of infection in an infected host, support viral shedding, or protect uninfected hosts exposed to the virus, therefore, this antibody, used alone or in combination, has the potential to prevent and treat COVID-19 and possibly other emerging human diseases caused by the subgenus Sarbecovirus, in addition to being a low-toxic drug and safe. Its use in these patients.
Keywords
Severe Acute Respiratory Syndrome; Viral pneumonia; Coronavirus infections; Antibodies; Cytokines; lymphocytes; Vaccines; COVID-19 Treatment
Introduction
The disease caused by a B-coronavirus, an RNA virus, had its
beginnings in 2019 in Wuhan - China where a series of cases of
pneumonia caused by this agent were identified, currently six
serotypes that produce diseases in humans are known., four
of these are prevalent and cause typical cold symptoms, the
remaining two are responsible for the appearance of severe
acute respiratory syndrome SarsCoV and Middle East respiratory
syndrome MERS-CoV; After nucleic acid sequencing of lower
respiratory tract epithelial cells from 4 patients with confirmed
pneumonia of unknown cause by real-time reverse transcription
PCR at Beijing Hospital, a novel 2019-nCoV beta coronavirus was
found, which was then was called SARS-CoV-2, also giving as a
result that this new virus has information from the subgenus
Sarbecovirus, of the Orthocoronavirinae family, which makes it
different from SARS-CoV and MERS-CoV, however according to
various reports it was identified that the genome of SARS-Co V-2,
is between 75% and 80% identical to SARS-CoV and therefore
its name, SARS-CoV-2 belongs to this genus of coronavirus and
its genome consists of a single-stranded RNA 29 kb in length.
The genome encodes 4 main structural proteins: the so-called
spike (S), membrane (M), envelope (E), and nucleocapsid (N).
The last protein is present inside the virion and is associated
with the viral RNA, and the other 3 proteins are associated
with the external structure of the virus, this disease causing
the current pandemic of global importance is characterized by
affinity to the respiratory system has characteristics important
as the production of increased pro-inflammatory cytokines and
a decrease in the response of T cells, which is directly related
to inflammation and severe lung damage that occurs in patients
infected with SarsCoV2, also highlighting the onset of given
symptoms from 5 days, the incubation period of approximately
14 days and the high transmission capacity of the virus given
by various mechanisms such as droplet transmission (given
when the infected person coughs or sneezes and these droplets
released by this mechanism are inhaled by the people nearby), by
contact (when an individual has direct contact with contaminated
surfaces). inhaled and then passes these through the eyes
and mouth) and by aerosols (this occurs when the respiratory
droplets of the infected are in contact with the environment in
places with little ventilation or closed that when inhaled cause
infection), in addition From this, manifestations and alterations
have been found in the gastrointestinal area, since enterocytes
with high expression of ACE II receptors have been found in this
area, which is why fecal transmission is also described in a smaller
proportion and less frequently (1). The most affected patients
are individuals between 25 and 50 years of age with a mean of
49 years, affecting mostly men and immunosuppressed patients
and with the presence of comorbidities such as diabetes mellitus,
arterial hypertension, renal dysfunction, liver dysfunction and
abnormalities. At the cardiovascular level, there are predominant
symptoms of fever, dyspnoea, gastrointestinal manifestations
(diarrhoea, decreased appetite, nausea and vomiting), cough,
anosmia (loss of smell) secondary to alterations at the level
of the olfactory nerve, ageusia (loss of taste) conferred by the
high expression of ACE II receptors on the oral mucosa, fatigue,
tiredness, urticarial, rashes, acrocyanosis and in more severe
cases, septic shock, haemorrhages, coagulopathy and metabolic
acidosis may occur [1-3].
Currently there are only maintenance and symptom management
therapies, since there are no exact therapies for the management
of severe SarsCoV2 infection, antiviral drugs and other drugs
used to manage the infection such as acyclovir, panciclovir,
chloroquine are used , ganciclovir, remdesivir, nitazoxadine,
corticosteroids among other medications currently used for
symptom management. In addition to these management
alternatives, there is also the use of anti-monoclonal antibodies
for the treatment of covid-19, these monoclonal antibodies
against SARS-CoV-2 are divided into three groups: 1) antibodies
that inhibit the binding and viral entry by targeting virus-specific
structures or host receptors, 2) antibodies that interfere with
viral replication and/or transcription, and 3) antibodies that
block various steps of the immune system response [1, 4]. This
therapy is considered one of the innovative findings for the
management of this disease, approved by the Food and Drug
Administration (FDA), it is about antibodies obtained by means
of recombinant DNA technology and hybridoma, which have
a capacity to neutralize the receptor binding domain (RBD) [5, 6]. The production of antibodies depends on B cells and once
they come into contact with the antigen, these antibodies are identical in specificity, which is why they are called monoclonal
antibodies, since they are produced by a single type of B cells
from the same cloned or stem cells. Some monoclonal antibodies
are currently used to treat COVID-19, standing out in this group
itolizumab and tocilizumab, the group's mechanism of action
is based on the fact that monoclonal antibodies bind to target
molecules, which can be surface membrane receptors, proteins
associated with enzymatic systems or circulating proteins, which
produces direct or indirect effects on tissue function, where
virus neutralization occurs when a sufficient number of epitopes
(antigenic determinant is the portion of a macromolecule that is
recognized by the immune system, specifically the sequence to
which antibodies bind, B cell receptors or T cell receptors.) on the
surface of the virus are occupied by antibodies. The occupancy
model assumes that obtaining sufficient antibody density in
the virus is the most important factor in neutralizing the virus,
causing inhibition of binding to cell receptors or interfering with
the plasma membrane or endosome fusion process. In addition
to this, the antibodies neutralize the infection caused by the virus
through their effector function in the Fc region (crystallizable
fraction) which is specific for each virus [5, 7]. This alternative
represents a great therapeutic option for the management of
COVID-19, since it has been shown that it improves the clinical
evolution of infected patients, mostly evidenced in adults than,
in children, that is, it has use in the population that is affected by
most frequently by this agent [6-8]. The use of this neutralizing
antibody therapy can alter the course of infection in an infected
host, support viral shedding, or protect uninfected hosts
exposed to the virus, therefore this antibody, used alone or in
combination, has the potential to prevent and treat COVID-19
and possibly other emerging human diseases caused by the
Sarbecovirus subgenus, in addition to being a low-toxicity drug
and safe to use in these patients [9, 10].
Methodology
To carry out this article, a bibliographic search was carried out
in various databases such as Elsevier, Scielo, Medline, PubMed,
Science Direct and Ovid, thus selecting original articles, case
reports and bibliographic reviews from 2020 to 2022, in Spanish
and English. using MeSH terms: Severe Acute Respiratory
Syndrome; Viral pneumonia; Coronavirus infections; Antibodies;
Cytokines; lymphocytes; Vaccines; COVID-19 treatment and or.
Thus including all the documents that will deal with the use of
anti-mononuclear antibodies as a therapy for Covid-19, the data
found was between 16-28 records, thus using 22 articles for the
preparation of this document.
Results
Passive immunotherapy in convalescent plasma or monoclonal antibody preparations has been evaluated for the treatment of COVID-19. The trimetric spike glycoprotein on the viral surface is the main target of the antibody as the spike plays an essential role in allowing the virus to attach to and infect host cells. The SARSCoV- 2 spike glycoprotein is composed of S1 and S2 domains. The S1 domain contains the receptor-binding domain (RBD) that specifically binds to the cellular receptor for human angiotensinconverting enzyme 2 (ACE2), and some RBD-binding antibodies block the interaction between the RBD and the ACE2 receptor, which leads to neutralization of SARS-CoV-2 infection. Evidence has shown that potent neutralizing monoclonal antibodies that recognize viral RBD are often elicited in SARS-CoV-2 infection. In recent years, highly specific and neutralizing monoclonal antibodies have been successfully isolated against various viruses, serving as an advanced replacement for convalescent plasma in passive immunotherapy. These biologics are now being considered therapies to combat COVID-19 outbreaks. Monoclonal antibodies that target SARS-CoV-2 RBD are being evaluated in outpatients, and trial data suggest that an antibody cocktail of two antibodies, REGN10933 and REGN10987, administered together reduces viral load and related hospital visits. infections in patients with COVID-19 compared to placebo [11,12].
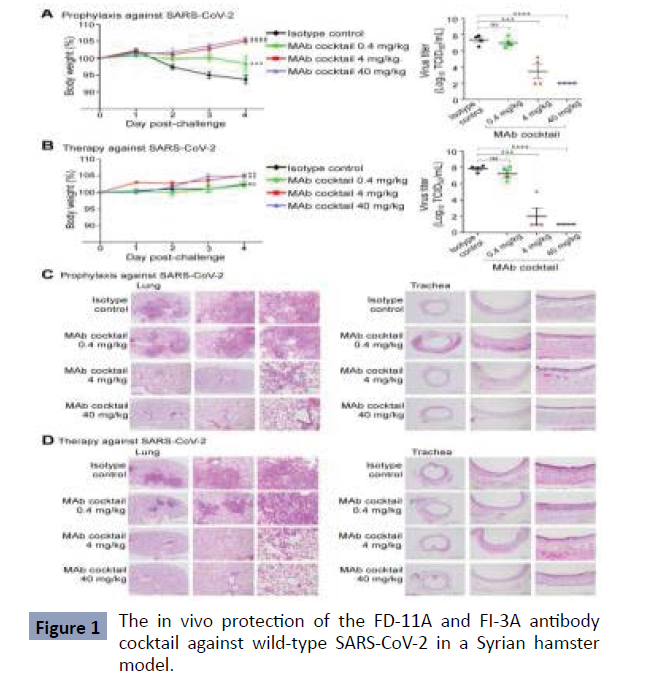
A study from Taiwan, published in 2022 found that representative antibodies targeting non-overlapping epitopes are effective against wild-type virus and recently emerging variants of concern, while encoded by antibody genes with few somatic mutations. Neutralization is associated with inhibition of viral RBD binding to ACE2 and possibly the subsequent fusion process. Structural analysis of representative antibodies, by cryo-electron microscopy and crystallography, reveals that they have some unique aspects that are of potential value while sharing some features in common with previously reported neutralizing monoclonal antibodies. The in vivo efficacy of an antibody cocktail composed of two potent non-competing anti-RBD antibodies was evaluated in a Syrian hamster model. We show that the cocktail prevents weight loss, reduces lung viral load, and attenuates lung inflammation in hamsters in both prophylactic and therapeutic settings. Although the neutralization of one of these antibodies is abrogated by mutations of the B.1.351 variant, it is also possible to produce a bivalent cocktail of antibodies both resistant to variants B.1.1.7, B.1.351 and B.1.617.2 and concluded that neutralizing antibodies target non-overlapping epitopes on the RBD of SARS-CoV-2, which function through mechanisms involving inhibition of receptor attachment and the fusion process. Combination of potent FD-11A and FI-3A exhibits both prophylactic and therapeutic efficacy in a hamster model of SARS-CoV-2 infection and serves as a promising therapeutic cocktail [11] (Figure 1).
Obtained from: Huang KA, Zhou D, Tan TK, et al. Structures and therapeutic potential of anti-RBD human monoclonal antibodies against SARS-CoV-2. Theranostics. 2022; 12(1):1-17. Published 2022 Jan 1. doi:10.7150/thno.65563
Figure 1 the in vivo protection of the FD-11A and FI-3A antibody cocktail against wild-type SARS-CoV-2 in a Syrian hamster model. A, The prophylactic effect of antibody cocktail at 40 mg/kg, 4 mg/kg, and 0.4 mg/kg. A single dose of antibody or control was administered intraperitoneally one day before intranasal virus challenge. b, The therapeutic effect of the antibody cocktail at 40 mg/kg, 4 mg/kg and 0.4 mg/kg. A single dose of antibody or isotopes control was administered intraperitoneally three hours after intranasal virus challenge. Body weight was measured at the indicated time points and the data normalized to the initial weight of each animal. Infectious viral loads in the lungs were measured by the tissue culture median infectious dose (TCID50) assay. Data represent the mean ± standard error of the mean (SEM) (n=4 per group). Anti-influenza The neuraminidase human IgG1 antibody Z2B3 was included as an isotype control. Statistical significance between groups was analyzed using twoway ANOVA and Turkey’s post hoc test. The results of the post hoc comparisons between the isotype control and the treatment group were shown in the graph. **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; ns, not significant. C,D, Histopathological findings of the lungs at (C) prophylactic and (D) therapeutic treatment of the antibody cocktail at 40 mg/kg, 4 mg/kg and 0.4 mg/kg in hamsters four days after infection. By SARS-CoV-2.
Figure 1: The in vivo protection of the FD-11A and FI-3A antibody cocktail against wild-type SARS-CoV-2 in a Syrian hamster model.
In a study conducted in the United States and published in 2022 using a systems analytic approach, they studied peripheral blood samples obtained from patients enrolled in a single institution in the SAC-COVID trial to discern the impact of CD24Fc treatment on the homeostasis. Performing high-dimensional spectral flow cytometry and measuring the levels of a wide range of cytokines and chemokine’s to discern the impact of CD24Fc treatment on immune homeostasis in COVID-19 patients; in this it was found that Twenty-two patients were enrolled and the clinical characteristics of the CD24Fc versus placebo groups were matched. Using high-content spectral flow cytometry and network-level analysis, we found that COVID-19 patients had systemic hyper activation. From multiple cellular compartments, including CD8+ T cells, CD4+ T cells, and CD56+ Natural Killer Cells. Treatment with CD24Fc mitigates this systemic inflammation, inducing a return to homeostasis in NK and T cells without compromising the antibody response against Spike protein. Significantly Attenuated CD24Fc Accelerated Systemic Cytokine Response and Decreased Cytokine Co-Expression and Network Connectivity Linked to COVID-19 Severity and Pathogenesis; therefore, they concluded that the data presented here offer immunological insights that underscore the encouraging clinical findings of the SAC-COVID trial. And these results strongly support further investigation of CD24Fc for various infamous conditions, including COVID19. CD24Fc has been tested in Phase II clinical trials to attenuate graft-versus-host diseases and showed promising efficacy, opening up the potential use of this drug in other immune-related diseases [12-14] (Figure 2).
Retrieved from: Song, NJ, Allen, C., Vilgelm, A.E. et al. Treatment with soluble CD24 attenuates COVID-19-associated systemic immunopathology. J Hematol Oncol 15, 5 (2022). https://doi. org/10.1186/s13045-021-01222-y
Figure 2 CD24Fc treatment down regulates systemic cytokine response in COVID-19 patients. Relative differences in plasma concentrations of cytokines/chemokine’s between HD (n=25) and patients with COVID-19 (n=22). Values were logarithmically transformed and evaluated using independent sample t-test. Significantly up- and down-regulated markers are shown (A). Heat map analysis (B) visualized the relative levels of cytokines/ chemokine’s (placebo: D1 n=12, D2 n=12, D4 n=11, D8 n=5; CD24Fc: D1 n=10, D2 n= 10, D4 n=9, D8 n=3). Using log-10 transformation of cytokine concentrations (dots) and GLMMpredicted fixed effect trends (lines), changes in IL-10 (C; p=0.05) and IL-15 (D; p=0.002) in CD24Fc (red) and placebo (black) groups were revealed. Values and trend lines cantered on mean D1. The p value was calculated using the Ken ward-Roger method. Cytokine score was analyzed longitudinally using a weighted sum approach (E; p<0.001). Using Pearson correlation matrices (F) and network maps (G; edge weight represents correlation coefficient), 30 HD plasma markers (n=25), COVID-19 reference (D1, n=22 ), placebo (pooled D2-D8, n = 28), and CD24Fc-treated groups (pooled D2-D8, n = 24) were visualized. Using these correlation coefficients, a density plot (H; D1 vs. placebo, p=0.07; D1 vs. CD24Fc, p<0.001; placebo vs. CD24Fc, p<0.001). The Kolmogorov- Smirnov test was used to evaluate equality of densities between groups. Connectivity analysis (I) and cytokine network centrality analysis (J) show cytokine expression relationships Network connectivity plots show highly correlated connections for each cytokine (i.e., node degree) and were evaluated using paired t-test. Cytokine network centrality analysis used the eigenvector centrality score that considers global network connectivity and correlation coefficients between cytokines (HD vs. D1, p<0.001; D1 vs placebo, p=0.08; D1 vs CD24Fc, p<0.001). Bartlett's test evaluated the significance of the variance of the centrality scores (HD vs. D1, p = 0.013; D1 vs. placebo, p = 0.17; D1 vs. CD24Fc, p = 0.008). Each point in I and J represents a cytokine. *p<0.05; **p<0.01; ***p<0.001
Figure 2: CD24Fc treatment down regulates systemic cytokine response in COVID-19 patients.
A study conducted in Iran, which was published in 2022, found that currently in terms of monoclonal antibodies, the main research has focused on exploring mAbs that target the pathogenic protein. The Te S protein is presented on the surface of the coronavirus and plays a vital role in virus entry and induces host immune responses. Previous studies have recognized a variety of mAbs effective in preventing the virus from entering host cells by targeting the SARS-CoV S protein. Depending on the binding site, antibodies that bind to the S protein can inhibit the conformational change of the S protein. protein S and block membrane fusion or block its interaction with ACE2. Therefore, protein S could be considered as a key target to develop effective mAbs against SARS-CoV-2. The critical target of mAbs is RBD (residues n318–510) of protein S [15, 16].
Discussion
Neutralizing monoclonal antibodies are therapeutic tools with
great potential for use, since they can be specifically directed to
the binding of the receptor (RBD, N318-V510) of protein S or it
has also been found that it can bind to the receptor protein ACE2
blocking viral entry in both cases [17], although the immune
response is a key factor for the response capacity of patients
infected with SARS-CoV-2 and even more so in those who suffer
from comorbidities or are in severe stages , as this response is
usually diminished, with evidence of lymphopenia and hyper
inflammation. It has been shown that age is a risk factor that plays
a relevant role related to immunosenescence; and that in young
people when there is a reinfection this can aggravate the disease,
therefore, the establishment of an adequate treatment in these
patients changes the prognosis of life in these age ranges.
The evidence on this type of medication is in continuous
development, on the one hand, due to the interesting mechanisms of action and the impact of this pandemic, but without ruling out
that the development in many cases of new molecules entails
commercial appeal, therefore, it is necessary to keep informed
of the new evidence without losing its critical vision and analysis.
In a trial named REMAP-CAP, 56 patients hospitalized for Covid
19 were randomized, of which 28 patients were assigned to the
sarilumab cohort and 28 were used as controls (standard care
treatment). The results report that by day 28, 61% of patients
treated with sarilumab experienced clinical improvement and
7% died. These findings were not significantly different from the
comparison group (clinical improvement 64%, mortality 18%)
[18].
In a meta-analysis comparing the efficacy of sarilumab versus
standard care alone or placebo, there is no evidence of a
statistically significant benefit on all-cause mortality at day 28
(RR 0.77, 95% CI 0.43 to 1 .36; 2 RCTs , 880 participants; low
certainty), on all-cause mortality at day 60 (RR 1.00, 95% CI 0.50
to 2.0; 1 RCT , 420 participants; low certainty ) and serious adverse
events (RR 1.17, 95% CI 0.77 to 1.77; 2 RCTs, 880 participants;
low certainty [19-22].
Taking this into account, monoclonal antibodies, due to their
effectiveness in preventing viral and bacterial infections, or those
mediated by toxins, as well as their use in the prevention and
post-exposure prophylaxis of pathologies in which the infectious
agent has an incubation period and prolonged replication,
adding its low rate of adverse effects, and finding ourselves in a
growing appearance of germs multi-resistant to antibiotics, it is
considered a therapeutic alternative for the treating physician,
taking into account that the aforementioned causes us to exhaust
the therapeutic alternatives in infectious diseases with high
morbidity and mortality, also taking into account that it must be
studied in depth to improve its specifications when establishing
its treatment.
Conclusion
The SARS-CoV-2 pandemic spread through human-human
transmission, and, although the virus identifies ACE2 receptors
in epithelial cells of various organs, it is possible that there are
increases in antibody-dependent infections (ADEs). Therefore,
this aspect should be considered when evaluating vaccines or
using monoclonal antibodies, however, the use of these has
presented a change in the treatment of Covid.
Antibodies are produced by the body as a defence against
disease. However, these can also be made in laboratories from
cells taken from people who have recovered from an illness.
Antibodies that are designed to treat or target a specific protein
(in this case, a protein from the covid-19 virus) are called
"monoclonal", which attach to the covid-19 virus and prevent
it from entering and replicate in human cells, which is intended
to help fight infection. Monoclonal antibodies have been used
successfully to treat other viruses.
An antibody is a protein that is produced naturally by the immune
system in response to an infection. A monoclonal antibody is a
molecule developed in a laboratory that is designed to mimic or
enhance the body's immune system's natural response against an invader, such as cancer or infection. Monoclonal antibodies
have an advantage over other types of infection treatment
because they are created to specifically target an essential part
of the infectious process. A monoclonal antibody is generated by
exposing a white blood cell to a particular viral protein, which is
then cloned to mass-produce antibodies that target that virus.
Before COVID-19, monoclonal antibodies were developed to
treat various viral infections, such as Ebola and rabies.
We found that the use of the antibody can be effective for the
elimination of SARS-CoV-2, in patients and even more so in
those with a lower initial immune response. The FDA approves
the use of these drugs under an emergency authorization in
adult patients and in pediatric patients (over 12 years of age)
who have moderate disease and who have a high risk of disease
progression to severe and that includes hospitalization, for this
the FDA highlights the established high risk criteria among which we find: obesity, chronic kidney disease, immunosuppressive
treatment, diabetes mellitus, age >65 years, age >55 years with
comorbidities and age 12-17 years with comorbidities (obesity,
congenital and/or acquired cardiovascular disease, sickle cell
anaemia, neurological disorders and asthma, or other respiratory
diseases that require daily drug treatment for control).
In the bibliography consulted, it was shown that the toxicity of
these drugs is low, and no evidence was found on their toxicity
during administration to patients with COVID-19. The evidence
on this type of medication is in continuous development, on
the one hand, due to the interesting mechanisms of action and
the impact of this pandemic, but without ruling out that the
development in many cases of new molecules entails commercial
appeal, therefore, it is necessary to keep informed of the new
evidence without losing its critical vision and analysis.
REFERENCES
- Vargas K, Schreiber V, Ochoa E, Lopez A (2020) SARS-CoV-2: una revision bibliographical de los temas mas relevantes y evolucion del conocimiento medico sobre la enfermedad. Neumol Cir Torax 79.
Indexed at, Google Scholar, Crossref
- Platelo T, Llocclla S, Guevara N (2020) terapia de plasma convalescent para pacientes con Covid-19: revision de la literature. Rev Fac Med Hum 4.
Indexed at, Google Scholar, Crossref
- Palacios M, Santos E, Velasquez M, Leon M (2020) COVID-19 una emergencia de salud publica mundial. Rev Clin Esp marzo
Indexed at, Google Scholar, Crossref
- Medina C (2021) Anticuerpos monoclonales: aplicaciones en infectologla. Rev Diagnostico 60.
Indexed at, Google Scholar, Crossref
- Jomarron Y, Serrano T, Pelaez R, Guirola J, Mastrapa H (2021) Plasma hiperinmune y anticuerpos monoclonales en el tratamiento de COVID-19, aspectos farmacologicos y toxicologicos, Rev. Toxicologica febrero.
Google Scholar
- Juarez G, Del Rio J, Arevalo A (2021) Anticuerpos monoclonales ante la COVID-19 Rev. El farmaceutico hospitals 221:10-17.
Indexed at, Google Scholar
- Bergheezan A, Suarez M (2020) tratamientos potenciales para covid-19 (infeccion por SARSCoV2), Rev. Associations espanola de pediatria de atencion primaria.
Google Scholar, Crossref
- Wang C, Li W, Drabek D (2020) A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun 11:2251.
Indexed at, Google Scholar, Crossref
- Galarraga F, Castro M, Viroga S (2021) Actualizacion sobre uso de anticuerpos monoclonales en Covid-19. Boletin farmacologicos.
Indexed at, Google Scholar
- Lozada I, Nunez P (2020) Covid-19: respuesta inmune y perspectivas terapeuticas Rev. Peru Med Exp Salud public 37.
Indexed at, Google Scholar, Crossref
- Huang KA, Zhou D, Tan TK (2022) Structures and therapeutic potential of anti-RBD human monoclonal antibodies against SARS-CoV-2. Theranostics 12:1-17.
Indexed at, Google Scholar, Crossref
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H et al. (2021) REGN-COV2 a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med 384:238-51.
Indexed at, Google Scholar, Crossref
- Song NJ, Allen C, Vilgelm A E (2022) Treatment with soluble CD24 attenuates COVID-19-associated systemic immunopathology. J Hematol Oncol 15,5
Indexed at, Google Scholar, Crossref
- Hofmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T et al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. 181:271–80.
Indexed at, Google Scholar, Crossref
- Niknam Z, Jafari A, Golchin A (2022) Potential therapeutic options for COVID-19: an update on current evidence. Eur J Med Res 27:6.
Indexed at, Google Scholar, Crossref
- Robson B (2020) COVID-19 Coronavirus spike protein analysis for synthetic vaccines, a peptidomimetic antagonist, and therapeutic drugs, and analysis of a proposed achilles’ heel conserved region to minimize probability of escape mutations and drug resistance Comput Biol Med.
Indexed at, Google Scholar, Crossref
- Zhou G, Zhao Q (2020) Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS-CoV-2. Int J Biol Sci 16:1718-1723.
Indexed at, Google Scholar, Crossref
- Gordon AC, Mouncey PR (2021) REMAP-CAP Investigators Interleukin-6 receptor antagonists in critically ill patients with COVID-19 N Engl J Med.
Indexed at, Google Scholar, Crossref
- Ghosn L, Chaimani A, Evrenoglou T, Davidson M, Grana C et al. (2021) Interleukin-6 blocking agents for treating COVID-19: a living systematic review. Cochrane Database. Syst Rev 3:CD013881.
Indexed at, Google Scholar, Crossref
- Owji H, Negahdaripour M, Hajighahramani N (2020) Immunotherapeutic approaches to curtail COVID-19. Int Immunopharmacol 88:106924.
Indexed at, Google Scholar, Crossref
- Hurt AC, Wheatley AK (2021) Neutralizing Antibody Therapeutics for COVID-19. Viruses 7 de abril de13.
Indexed at, Google Scholar, Crossref
- Tuccori M, Ferraro S, Convertino I, Cappello E, Valdiserra G et al. Anti-SARS-CoV-2 neutralizing monoclonal antibodies: clinical pipeline.
Indexed at, Google Scholar, Crossref
Citation: Bello Cordero JF, Benitez Russo
UD, Torres Hernandez FA, Moreno Martinez
JM, Carmen Mercado Oliva MD, et al. (2022)
Use of Anti-Mononuclear Antibodies as
Therapy for Covid-19. Health Sci J. Vol. 16 No.
S7: 948.