Key words
Danio rerio, Nonylphenol, T-Box, no tail, Spade tail, Quantitative gene expression, Embryonic development
Özet: 353-Nonylfenolün Zebra Bal??? Embriyolar?nda T-BOX6 Geninin Ekspresyonuna Neden Olmas? ve Bunun, Deformitelerle Tan?msal Bilgiyle ?li?kilendirilmesi
Nonylpehoneller (NP) dünya genelinde çevremizde bulunurlar. Teknik olarak NP kar???m?n? olu?turan ana bile?en p353-NP, zebra bal?klar?nda (Danio rerio) embriyonik geli?me bo-zuklu?una neden olmaktad?r. Zebra bal??? embriyolar?nda d?? görünü? olarak ay?rt edilebilen fenotipler knock-out veya di?er mutantlar sayesinde elde edilir. Bu çal??malarda kullan?lan genler: kuyruksuz (ntl) geni, mahmuz kuyruk (spt) ve t box 6 (tbx6) genidir. Çal??man?n amac? p353-NP’nin normal kuyruk geli?iminde T-box ailesine mensup genlere olan teratogenik etkisini inceleyerek NP kaynakl? hastal?klar?n mekanizmalar?n?n daha iyi anla??lmas?d?r. Zebra embriyolar? sub-lethal konsantrasyonda NP izomerleri ile muamele edilmi?tir. Özel bir fenotip olan kuyruk ucunda a??r? ?i?me sadece p353-NP kullan?ld???nda görülmü?tür. Ntl geni ve spt geninin PCR ile say?m? sonucu de?i?memi? olmakla birlikte tbx6 geninde p353-NP sonras? a??r? art?? görülmü?tür.
Anahtar Kelimeler:
Danio rerio, Nonylphenol, T-Box, kuyruksuz, Mahmuz kuyruk, Kantitativ gen ekspresiyonu, Embriyonik geli?im
Introduction
Nonylphenols (NP) have been found in almost every environmental compartment such as rain and surface water, snow, sewage sludge and sediments (Lin et al, 2004; Kawahata et al. 2004; , 2006; Stachel et al. 2005; Isobe et al. 2004; Kannan et al. 2003). NP are microbiological me-tabolites of alkylphenol polyethoxylates. Al-kylphenol polyethoxylates have exceptional sur-face properties, are used widely in industrial pro-cesses, and account for 5% of the tenside market (Sharma et al. 2009).
NP directly interact with the estrogen receptor in vertebrates (Owens et al. 2003; Folmar et al. 2002; Bevan et al. 2003) and exert effects on the reproduction of fish especially in vitellogenin synthesis (Harries et al 1997, Christiansen et al. 1998, Giesy et al. 2000) – a precursor of lipo-proteins and phosphoproteins in egg yolk-pro-duction. Additionally, NP disturb early differen-tiation in fish inducing development of intersex gonads and circulatory and other systemic ab-normalities (Gray et al. 1997). NP bind to the 17-β-estradiol receptor, induce morphologic defor-mations, increase apoptosis, and alter the deposi-tion and differentiation of neural crest-derived melanocytes in the tailbud stage of X.laevis em-bryos (Bevan et al 2003). Their tendency to partly mimic estrogen and disrupt the natural hormonal balance supports the inclusion of NP to the group of endocrine disrupters. A detailed analysis of the risk posed by NP was conducted by the European Union and led to restricted use within the EU (ECB 2002).
Due to the synthetic methods used, technical NP (t-NP) is a mixture of approximately 20 para-substituted isomers with differently branched al-kyl chains (Russ et al. 2005; Wheeler et al. 1997) whereas isomeric composition varies between mixtures p353-NP is the most abundant isomer. The absolute concentration of p353-NP in tech-nical mixtures depends on the production process (Russ et al. 2005). It was shown previously that NP isomers varied in their estrogenic potency in the Yeast Estrogen Screen (YES) assay (Gabriel et al. 2008), in the E-screen (Preuss et al. 2010) as well as the MVLN reporter gene cell assay (Preuss et al. 2006, Preuss et al. 2010) demon-strated that t-NP and p353-NP, as well as p363-NP, p33-NP and p252-NP can be classified as partial agonists in the MVLN cell assay, whereas p262-NP, p22-NP and 4n-NP can be classified as antagonists in the MVLN cell assay.
Embryos of zebrafish (Danio rerio) have of-ten been used in toxicity studies of environmen-tally relevant substances (Hollert et al. 2003; Kosmehl et al. 2006; Scholz et al. 2008). An ad-vantage of zebrafish as test organism in toxico-logical research is the transparency of the eggs. Spinal malformation observed after exposure of zebrafish embryos to p353-NP (Kammann et al. 2009) is comparable to those reported form sev-eral groups working on developmental processes: Zebrafish expressing a mutant (non-functional) of the no tail (ntl) gene or a mutant of the spade tail gene (spt, tbx16) lack the notochord and tail (Halpern et al. 1993, Amacher et al. 2001; Schulte-Merker et al. 1994). Studies with knock out mutants showed that homozygous spt—(dou-ble knock out embryos) lack trunk somites and trunk muscle later in development (Griffin et al. 1998; Ho et al. 1990). Ntl and spt proteins belong to the T-box6/16 protein subfamily of T-box transcription factors which contain a DNA bind-ing and protein dimerization domain: The T-box (Naiche et al. 2005). The T-box6/16 subfamily has important functions in the development of the mesoderm in all mammals investigated (Wardle et al. 2008). The damages of ntl gene knock out and spt gene knock out phenotypes in zebrafish are less severe than mutations in or depletion of the homologous genes in other vertebrates indi-cating a functional redundancy among zebrafish T-box genes.
Understanding the molecular mechanisms in-volved in misdevelopment produced by NP is a prerequisite for further investigations in limiting the effects of NP release to the environment. Aim of the present study was to correlate the observed tail deformation produced by p353-NP with ex-pression data of genes of the T-box6/16 subfam-ily. Zebrafish embryos were exposed to sublethal concentrations of different isomers of NP and real time PCR analysis for genes belonging to the T-box6/16 family was performed.
Material and Methods
Chemicals
The synthesized NP used in this study (Table 1) were synthesized and purified as described in Preuss et al (2006). All isomers had a purity >99%. Technical nonylphenol (t-NP) and dime-thyl sulfoxide (DMSO 99.9%) was purchased from Sigma-Aldrich/Fluka (Deisenhofen, Ger-many). Substances used for RNA extraction were purchased from AppliChem (Darmstadt, Ger-many), enzymes used for cDNA synthesis and real time PCR were supplied by MBI Fermentas (St. LeonRot, Germany).
Breeding of zebrafish
250 zebrafishes were held in breeding groups of 20 females and 30 males. Fish were kept at 26°C and a light/dark period of 14h/10h in tap water. For collection of eggs translucent plastic spawning boxes (12 x 20 x 12 cm) with a stain-less steel mesh insert (3 - 4 mm mesh size) were placed in the aquaria. On top of the mesh some green plastic net material and green glass marbles animated the fish to spawn into the box. Alt- hough not all fish spawned into the boxes, up to 3000 eggs/day could be collected from the stock using 2 boxes for each breeding group. Eggs are 1.0 - 1.2 mm in diameter and have a transparent chorion. Fertilized and well developed eggs in the 4 to 8 cell stage were selected for the test and placed in 24-well dishes (Nunc GmbH, Wiesba-den, Germany). The mean spontaneous lethality rate was 2%. Fertilization rates were > 80%.
Treatment of zebrafish
(1) For morphological effects observation zebrafish embryo test was carried out according to 31: In each well of a 24well dish 5 zebrafish embryos (4-8-cell stage) were exposed for 48 hr at 26°C to 1 ml test solution containing one NP isomer (2.5-50 μmol/L) and 1% DMSO resulting in a total number of 60 embryos for each test, ei-ther exposure or control. Since all experiments were repeated twice a total of 120 zebrafish em-bryos were used for each concentration of each chemical. Negative controls were exposed to the 1% DMSO solvent only. EC50 was calculated from concentration-effect curves for the spinal malformations. Measurement was the amount of embryo showing any spinal deformation without respecting a certain degree of the misdevelop-ment. Four-parameter logistic curves were used to describe the concentration-effect relation. From these, EC50 values were calculated.
(2) For RT-PCR 3-4 hr old zebrafish em-bryos were placed into 24-well dishes (5 embryos per well). For each experiment, either exposure or control, 30 embryos were used. The embryos were treated with the indicated NP at a final con-centration of 10 μmol/L for 1 hr in water con-taining DMSO at 26 °C. These parameters were chosen because in preliminary experiments ex-pression effects were observable after 1 hr and the concentration of NP were selected on the ba-sis of our previous study (Kammann et al. 2009). After incubation, the embryos were washed in fresh water and subsequently shock frozen in liq-uid nitrogen. All experiments - covering 6 NP isomers, the technical NP mixture (Table 1) and a negative control with DMSO only - were re-peated 5 times at different days. The concentra-tion of the test substances was 10 μmol/L, i.e., in the range of EC10 - EC50 of acute morphological effects of NP isomers in zebrafish after 48h ex-posure (Kammann et al. 2009).
RNA Extraction
RNA was extracted using a modification of the method of Chomczynski P, Sacchi (1987). In brief, 30 frozen embryos were carefully homoge-nized in 1 ml of GuaSCN-buffer (3 M guani-dinthiocyante, 50 mM TRIS, 10 mM EDTA, 8% sarcosyl, 1% β-mercapethanol pH 7.0). After ad-dition of 0.1 ml of 2 M sodium acetate (pH 4), 1 ml water saturated phenol and 0.2 ml chloroform the samples were centrifuged at 15.000g for 15 min. The supernatant was collected and RNA was precipitated by adding of 1 ml of isopropanol followed by two ethanol (70%) washes. Concen-tration and purity of RNA was determined by ab-sorption measurement at 230, 260 and 280 nm.
cDNA Synthesis
Two hundred ng total RNA was digested with DNase for 30 min at 37°C, terminated by adding EDTA to final a concentration of 25μmol/l) and then transcribed. Reverse transcription was car-ried out in a total volume of 20 μl containing 10 mmol/l Tris-HCl, (pH 8.8), 50 mmol/l KCl, 5 mmol/L MgCl2, 1 mmol/l each dNTPs, 0.5 μg oligo (dT) primers, 0.5 μg random primers, 25 U RNase inhibitor, and 200 U M-MuLV reverse transcriptase for 5 min at 25°C followed 1 hr at 42°min. The reaction was stopped by incubation for 5 min at 70°C
Real time PCR
Real time PCR was performed in an Applied Biosystems 7500 Real-Time PCR System using 1 μl of the synthesized cDNA in a final volume of 15 μl. Details on the primer combinations are shown in Table 3. Each assay included a no tem-plate control and all measurements were per-formed in duplicate. The efficiency of the system was measured by using serial 2-fold dilutions of a mixture of different cDNAs. The efficiency indi-cates the amplicon doubling rate of a primer pair. The Ct value is defined as the number of cycles needed for the fluorescence signal to rise above a threshold level of detection. Obtained Ct values of the dilution series were plotted and the result-ing slope of the linear graph was used for calcu-lation of the system efficiency using the equation: E=1/101/slope (Scheffe et al. 2006) The system ef-ficiency ranged between 1.91 and 2.11 (Table 2). The dissociation plots (melting curve analysis) indicated a single peak for all primer pairs further supporting specificity. To evaluate the different housekeeping genes used in the study the original concentration of the transcript was calculated using the formula Conc trans= E-Ct . The concen-tration of the transcripts ntl, spt and tbx6 related to the housekeeping genes ef1α and rpl13a was calculated using the formula:
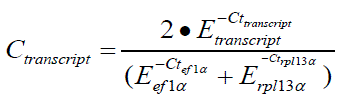
The concentrations of the transcript (arbitrary units) were multiplied by a transcript-specific factor to gain a scale from 0 to 100. These con- centrations are comparable within one transcript but not between transcripts.
Statistics
Statistical significances of relative mRNA ex-pression between different NP isomers were de-termined using Prism®4.03 (GraphPad Software, La Jolla, CA USA). The calculated concentrations related to the expression of rpl13a and ef1α were log transformed and univariate analyses of vari-ance (ANOVA) were performed to detect statisti-cally significant differences between any groups. Data are expressed as mean ±standard error of the mean (SEM), arbitrary units, values related to the expression of the gene of interest in the DMSO control and related to the mean of the expression observed with rpl13a gene and ef1α gene. As post test to determine the actual differences between individual groups the Tukey-Kramer test was em-ployed. The p-value of p<0.05 was considered to be significant.
Results and Discussion
The development of zebrafish embryos after incubation with various isomers of NP is demon-strated in Figure 1. Normal development after 48 hr of incubation results in a long extended tail without any bending or tip broadening (Figure 1H). The malformations in development of the tail produced by treatment with different isomers of NP are shown in Figure 1A-G. In some cases the malformation were accompanied by necrosis of the tail tip (results not shown). All NP isomers produced spinal malformations: Zebrafish em-bryos treated with p22-NP showed short tails which appeared to be incomplete developed (Figure 1A). Exposure of zebrafish embryos to p252-NP, p262-NP, p33-NP and t-NP results in bended tails. These tails showed a sharp kink in the middle of the tail length combined with a turn in the tail orientation (Figure 1B, C, D and G). Since the concentration of p22-NP and p33-NP required to induce the malformation described was 50μmol/l, calculation of EC50 was not pos-sible. p353-NP produced a tail tip broadening (Figure 1E) which was described previously (Kammann et al 2009). At a concentration of 16 μmol/l 50% of the embryos showed that defor-mation (Table1). Slight swelling of the tail tip was observed after exposure of embryos to p363-NP (Figure 1F). The phenotype of tail tip broad-ening is unique for p353-NP and was never seen with other isomers of NP. After 1 hr of treatment of 3-4 hr old embryos no marked differences in phenotypes could be detected. The first time when tail tip shape was visible was around 24 hr after fertilization – independent of the treatment. No general alterations in the tail were observed from then until 48 hr after fertilization, when the tail tip was visible best. This time frame was se- lected because in preliminary experiments one could demonstrate that a change in expression of the selected genes is observable after 1 hr expo-sure, demonstrating that gene activation of tran-scription factors is rapid.
To combine the effects of NP with expression data, several possible housekeeping genes used for quantification were investigated. The meas-ured concentrations of the housekeeping genes rpl13 (mitochondrial), ef1α and β-actin based on the amount of RNA used for reverse transcription is shown in Figure 2. No significant differences were found. However, the expression of β-actin gene appeared smaller in the p262-NP/ p22-NP/ p252-NP group compared to the other groups (p33-NP/ p353-NP/ p363-NP, t-NP and DMSO alone). Zebrafish embryos treated with NP meth-ylated on the 2’ position (p22-NP, p252-NP, p262-NP) compared with zebrafish embryos treated with NP methylated on the 3’ position (p33-NP, p353-NP, p363-NP) showed a mean difference of 0.47 (relative expression). This dif-ference failed to be statistical significant. As-signing the data of the NP known for antiestro-genic effects (p22-NP and p262-NP,) to one group and data for tNP known to exert estrogenic effects (p33-NP, p252-NP, p353-NP and p363-NP) to the other group reveals that the expression of β-actin gene in the antiestrogenic group is sig-nificantly different from the DMSO control while the comparison between the estrogenic group and DMSO control shows no significant differences (0,07±0.04 and 0,54±0.17 respectively). How-ever, since no marked differences were observed using the other housekeeping genes all further data were related to the mean of the expression observed with rpl13a gene and ef1α gene. This combination shows a homogeneous distribution over all groups (Figure 2d) and was used to nor-malize the expression data.
Expression of ntl gene, spt gene and tbx16 gene is shown in Figure 3. Although the zebrafish were harvested within a short period of time the variation within the single groups is remarkably high. Therefore statistical significant differences are seldom observed. Highest expression of ntl gene (38.8±30.6) was seen after exposition the embryos to p252-NP, followed by the expression after treatment with p33-NP (16.8±11.7). The ex-pression of ntl gene in the other zebra fish em-bryos varied between 7.7±4.4 in p22-NP and 0.8±0.6 in the p353-NP group. Expression of spt gene was highest in zebrafish embryos treated with t-NP embryos (33.3±15.6). The spt gene ex-pression in zebra fish embryos under treatment of the different isomers of nonyphenol used varied between 0.72±0.63 (p353-NP) and 20.9±16.1 (p252-NP). Performing linear regression using the expression of spt gene and ntl gene based on the response of the individual embryo revealed a significant correlation with a coefficient of de-termination R2= 0.9172.
The expression of tbx6 gene measured for zebrafish embryos exposed to all isomers but p353-NP and t-NP is comparable to the expres-sion observed in zebrafish embryos treated with DMSO (values between 0.3±0.1 for p252-NP and 2±2 for p33-NP). Zebrafish embryos treated with t-NP showed an expression of 9.8±3.7. A marked increase in tbx6 gene expression was observed in zebra fish embryos treated with p353-NP (83.5±14.7). This induction is statistically sig-nificant different from the expression of tbx6 gene in zebrafish exposed to DMSO alone.
In addition to the often described action of NP as estrogen like substances it was possible to demonstrate that some NP have the potential to induce tail-malformation in fish embryos. The observed tail malformation seen in our experi-ments may be due to miss-migration of precursor cells of the mesoderm in early development. The correct migration is a prerequisite of normal de-velopment (Christ et al. 2002). The various spinal malformations induced by NP isomers underline the different effects and possible modes of action known for NP isomers. p353-NP showed a unique morphological alteration: A broadened tail tip. This deformity might be related to the tbx6 expression as discussed below.
To investigate gene expression pattern of the tbx6/16 family in zebrafish embryos it is neces-sary to study the role of the housekeeping genes which might be regulated depending on devel-opmental stage and/or treatment of the organism (Guenin et al. 2009). Data showed found that the often used expression of housekeeping gene ß-actin gene is not suitable in experiments using NP. HSU et al. (1987) previously demonstrated that the ß-actin gene is upregulated by estrogens, interestingly a down regulation was found in the two antiestrogenic NP isomers (p22-NP and p262-NP;Figure 2a). The partial estrogenic iso-mers show quantitative down regulation. This gene expression pattern, in line with the known mode of action for the isomers, indicates that at the selected time point gene expression react al-ready to NP exposure. It was decided to use the average expression of two genes, i.e. ef1α gene and rpl13a gene, to normalize our data. An effect of NP on two other housekeeping genes (ef1α, rpl13a) used in our study was not observed, alt-hough chemical regulation of ef1α gene was re- ported when incubation was prolonged over 96 hr (Mc Curley et al 2008). The short incubation time of 1 hr in our study is probably the reason for our different observations.
Although the variation of the measure expres-sion within the groups is not small, the correla-tion between the measured values for spt gene and ntl gene is remarkable high. This indicates that the observed variation is not due to experi-mental conditions but to different stages in de-velopment of the used embryos. However, the effects of different isomers of NP on the expres-sion of transcription factors of the T-Box family in zebrafish embryos presented here shows that the increased expression of tbx6 gene was associ-ated with the malformation of the tail produced by p353-NP (Kammann et al. 2009). A numerical increase in tbx6 gene expression was also observ-able by using t-NP. This rise is only 1/10 of the increase observed with p353-NP (Fig 3c). The main components of t-NP are two diasteromeric forms of p353-NP with combined concentrations between 12.2 and 20 % (Russ et al. 2005; Katase et al. 2008; Eganhouse et al. 2009). Given that still several of the components of t-NP are not identified so far, and that some isomers of NP act estrogenically while other act antiestrogenically (Preuss et al. 2010) the expression of tbx6 gene as reaction of incubation the zebrafish embryos with t-NP is probably due to the p353-NP present in t-NP. The other genes belonging to the T-box6/16 subfamily (spt, ntl) are not fundamen-tally regulated by p353-NP within the incubation time of 1hr (Figure 3a,b). However, it needs to be emphasized that these observations are not a proof for a cause effect relationship, which fur-ther experiments may provide.
The family of T-Box genes have been investi-gated using knock out zebrafish with single knock out and multiple knock outs. Characteriza-tion of spt knock out, ntl knock out and ntl double knockout embryos shows that spt protein and ntl protein function in a partially redundant manner (Amacher et al. 2002). The regulation of the ex-pression of these genes is apparently similar, as a high correlation in the expression values of spt gene and ntl gene was found, Spt protein, ntl protein and tbx6 protein might interact in three ways: (a) two factors activate target genes that neither factor can activate on its own (combina-tory interaction), (b) two factors contribute to the activation of a gene in an additive manner and (c) one factor prevents activation of a gene by an-other factor (competitive antagonism). Summa-rizing the studies on gene expression in zebrafish regarding the T-box 6/16 subfamily and the demonstrated increase of tbx6 mRNA due to p353-NP exposure; it is most likely that tbx6 protein is abundant when zebrafish embryos are treated with p353-NP. It is presumed that the tbx6 protein competes with spt protein and an-tagonized the spt protein effect, resulting in sim-ilar phenotype (deformed tail) as spt knock out zebrafishes. This postulation is supported by the fact that tbx6 protein compete effectively with ntl protein promoted expression of some T-site de-pendent gene transcription (Goering et al. 2003).
It is not known which molecular mechanisms regulate the expression of tbx6 gene. Thus far only regulation via spt/ntl proteins is described: Tbx6 gene contains six spt/ntl protein binding sites within 500 bp of the transcription site, and expression-driven by a 500 bp tbx6 gene promo-tor is mainly regulated by spt protein and ntl protein, spt protein exerting more influence (Gar-net et al. 2009). In our experiments an over ex-pression of spt gene or ntl gene was not observed in embryos treated with p353-NP compared to the other NP isomers or the controls. The regula-tion of tbx6 gene in vivo is probably more com-plex with more regulatory elements in greater distance to the transcription site and not yet ana-lyzed by reporter gene experiments.
Gene expression levels of ntl (a), spt (b) and tbx6 (c) relating to the housekeeping genes rpl13α and ef1α of zebra fish embryos treated with different synthetic isomers of nonylphenols (NP) with a concentration of 10 Nmol/L for 1 hr. Technical NP (t-NP) is a mixture of more than 20 NP isomers. Dimethyl sulfoxide (DMSO) was used as vehicle control. Values are means of 5 independent experiments with SEM. Asterisks indicate significant difference (p<0.05) as compared to DMSO control (Tukey-Kramer test).
Conclusion
It was necessary to investigate gene expres-sion of tail formation in an early stage of embry-onic development, whereas the fully developed tail was visible considerably later. The develop-mental stage of 48 hr was selected to visually in-vestigate the embryos, because the visibility of the tail tip was best at this time. It is in general not possible to investigate gene expression during migration of precursor cells of the mesoderm and phenotype of tail tip formation at the same time. It was therefore necessary to combine two ex-periments conducted a different time points. One could detect reasonable results for ß-actin gene regulation at the time point select for investigat-ing the gene expression. This indicates that changes in gene expression due to NP might be investigated at the selected time point. As dis-cussed above, the observed relation of p353 NP and tbx6 gene expression is plausible as cause and effect, but this can not be proven with the shown experiments alone. With this investigation a short snapshot in embryonic gene expression was covered. Other genes related to NP toxicity might be expressed in different time frames. The combination of gene knock out experiments to analyze phenotypes induced by administration of NP may reveal new insights into the biological mechanisms of these xenobiotics. With our in-vestigation the starting point for such future ex-periments is provided. Only this hypothesis driven approach shed some light on the tran-scriptions factors initial responsible for gene ac-tivation or gene inactivation and in conclusion for the mode of action of NP in fish embryos.
Acknowledgment
The skilful technical assistants of Silvia Kaschner and Dominik Jasinski are acknowl-edged. This work has been supported by the Fed-eral Ministry of Food, Agriculture and Consumer Protection.
551
References
- Amacher, S.L., Draper, B.W., Summers, B.R., Kimmel, C.B., (2002). The zebrafish T-box genes no tail and spadetail are required for development of trunk and tail mesoderm and medial floor plate, Development, 129(14): 3311-3323
- nBevan, C.L., Porter, D.M., Prasad, A., Howard, M.J., Henderson, L.P., (2003).Environmental estrogens alter early development in Xenopuslaevis, Environ-mental Health Perspectives, 111(4): 488-496.doi: 10.1289/ehp.5500
- nChomczynski, P., Sacchi, N., (1987). Single-step method of RNA isolation by acid guanidiniumthiocyanate-phenol-chloroform extraction, Analytical Biochemistry, 162(1): 156-159.doi: 10.1016/0003-2697(87)90021-2
- nChrist, B., Brand-Saberi, B., (2002). Limb muscle development, International Journal of Developmental Biology, 46(7): 905-914
- nChristiansen, T., Korsgaard, B., Jespersen, A., (1998). Effects of nonylphenol and 17 beta-oestradiol on vitellogenin synthesis, testicular structure and cytology in male eelpout Zoarcesviviparus, Journal of Experimental Biology 201(2): 179-192
- nE.C.B., (2002). European Union Risk Assessment Report 4-nonylphenol(branched) and nonylphenol, European Commision, 10
- nEganhouse, R.P., Pontolillo, J., Gaines, R.B., Frysinger, G.S., Gabriel, F.L.P., Kohler, H.E., Giger, W Barber, L.B., (2009). Isomer-specific determination of 4-nonylphenols using comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry, Environmental Science and Technology, 43(24): 9306-9313.doi: 10.1021/es902622r
- nFolmar, L.C., Hemmer, M.J., Denslow N.D, Kroll, K., Chen, J., Cheek, A., Richman, H., Meredith, H., Grau, E.G., (2002). A comparison of the estrogenic potencies of estradiol, ethynylestradiol, diethylstilbestrol, nonylphenol and methoxychlor in vivo and in vitro, Aquaict Toxicology, 60(1-2):101-110.doi: 10.1016/S0166-445X(01)00276-4
- nGabriel, F.L.P., Routledge E.J., Heidlberger, A., Rentsch, D., Guenther, K., Giger, W., Sumpter J.P., Kohler, H.P.E., (2008). Isomer-specific degradation and endocrine disrupting activity of nonylphenols, Envi-ronmental Science and Technology, 42(17): 6399-6408
- nGarnett, A.T., Han, T.M, Gilchrist, M.J., Smith J.C., Eisen, M.B., Wardle, F.C., Amacher, S.L., (2009). Identification of direct T-box target genes in the developing zebrafish mesoderm, Development, 136(5): 749-760.doi: 10.1242/dev.024703
- nGiesy, J.P., Kannan, K., Blankenship, A.L., Jones, P.D., (2000). Hilscherova K. Dioxin-like and non-dioxin-like toxic effects of polychlorinated biphenyls (PCBs): implications for risk assessment, Central European Journal of Public Health, 8 Suppl: 43-45
- nGray, M.A., Metcalfe, C.D., (1997). Induction of testis-ova in Japanese medaka (Oryziaslatipes) exposed to p-nonylphenol, Environmental Toxicology and Chemistry, 16(5): 1082-1086
- nGriffin, K.J.P., Amacher, S.L., Kimmel, C.B., Kimelman, D., (1998). Molecular identification of spadetail: regulation of zebrafish trunk and tail mesoderm formation by T-box genes, Development, 125(17): 3379-3388
- nGoering, L.M., Hoshijima, K., Hug, B., Bisgrove, B., Kispert, A., Grunwald, D.J., (2003). An interacting network of T-box genes directs gene expression and fate in the zebrafish mesoderm, Proceedings of the National Academy of Sciences, 100(16): 9410-9415.doi: 10.1073/pnas.1633548100
- nGuenin, S., Mauriat, M., Pelloux, J., Van Wuytswinkel, O., Bellini, C., Gutierrez, L., (2009). Normalization of qRT-PCR data: the necessity of adopting a systematic, experimental conditions-specific, validation of references, Journal of Experimental Botany, 60(2): 487-493.doi: 10.1093/jxb/ern305
- nHalpern, M.E., Ho, R.K., Walker, C., Kimmel, C.B., (1993). Induction of Muscle Pioneers and Floor Plate Is Distinguished by the Zebrafish No Tail Mutation, Cell, 75(1): 99-111
- nJule, E., Harries, J.E., Sheahan, D.A., Jobling, S., Matthiessen, P., Neall, P., Sumpter, J.P., Tylor, T., Zaman, N., (1997). Estrogenic activity in five United Kingdom rivers detected by measurement of vitellogenesis in caged male trout, Environmental Toxicology and Chemistry, 16(3): 534-542.doi: 10.1002/etc.5620160320
- nHo, R.K., Kane, D.A., (1990). Cell-Autonomous Action of Zebrafish Spt-1 Mutation in Specific Mesodermal Precursors, Nature, 348(6303): 728-730.doi: 10.1038/348728a0
- nHollert, H.K.S., König, N., Rudolf, M, Ulrich, M., Braunbeck, T., (2003). A new sediment contact assay to assess particle-bound pollutants using zebrafish (Daniorerio) embryos, Journal of Soil Sediment, 3(3): 197-207 doi: 10.1065/jss2003.09.085
- nHsu, C.Y., Frankel, F.R., (1987). Effect of estrogen on the expression of mRNAs of different actin isoforms in immature rat uterus. Cloning of alpha-smooth muscle actin message, Journal of Biological Chemistry, 262(20): 9594-9600
- nIsobe, T., Takada, H., (2004). Determination of degradation products of alkylphenolpolyethoxylates in municipal wastewaters and rivers in Tokyo, Japan, Environmental Toxicology and Chemistry, 23(3):599-605.doi: 10.1897/03-263
- nKannan, K., Keith, T.L., Naylor, C.G., Staples C,A., Snyder, S.A., Giesy, J.P., (2003). Nonylphenol and nonylphenolethoxylates in fish, sediment, and water from the Kalamazoo River, Michigan. Archives of Environmental Contamination and Toxicol-ogy, 44(1):77-82.doi: 10.1007/s00244-002-1267-3
- nKammann U, Biselli S, Huhnerfuss H, Reineke N, Theobald N, Vobach M, Wosniok W (2004). Genotoxic and teratogenic potential of marine sediment extracts investigated with comet assay and zebrafish test, Envi-ronmental Pollution, 132(2): 279-287.doi: 10.1016/j.envpol.2004.04.021
- nKammann, U., Vobach, M., Wosniok, W., Schaffer, A., Telscher, A., (2009). Acute toxicity of 353-nonylphenol and its metabolites for zebrafish embryos, Envi-ronmental Science and Pollution Research, 16(2): 227-231.doi: 10.1007/s11356-008-0097-x
- nKatase, T., Okuda, K., Kim, Y.S., Eun, H., Takada, H., Uchiyama, T., Saito, H., Makino,. M., Fujimoto, Y., (2008). Estrogen equivalent concentration of 13 branched para-nonylphenols in three technical mixtures by isomer-specific determination using their synthetic standards in SIM mode with GC-MS and two new diasteromeric isomers, Chemosphere, 70(11): 1961-1972.doi: 10.1016/j.chemosphere.2007.09.049
- nKawahata, H., Ohta, H., Inoue, M., Suzuki, A., (2004). Endocrine disrupter nonylphenol and bisphenol A contamination in Okinawa and Ishigaki Islands, Japan--within coral reefs and adjacent river mouths, Chemosphere, 55(11): 1519-1527.doi: 10.1016/j.chemosphere.2004.01.032
- nKosmehl, T., Hallare, A.V., Reifferscheid, G., Manz, W., Braunbeck, T., Hollert, H., (2006). A novel contact assay for testing genotoxicity of chemicals and whole sediments in zebrafish embryos, Environmental Toxicology and Chemistry, 25(8): 2097-2106.doi: 10.1897/05-460R.1
- nLin, C., Spikings, E., Zhang, T., Rawson, D.M., (2009). Effect of chilling and cryopreservation on expression of Pax genes in zebrafish (Daniorerio) embryos and blastomeres, Cryobiology, 59(1): 42-47.doi: 10.1016/j.cryobiol.2009.04.007
- nLin, C.Y, Chen, Y.H., Lee, H.C., Tsai, H.J., (2004). Novel cis-element in intron 1 represses somite expression of zebrafish myf-5, Gene, 334:63-72.doi: 10.1016/j.gene.2004.03.016
- nMcCurley, A.T., Callard, G.V., (2008). Characterization of housekeeping genes in zebrafish: male-female differences and effects of tissue type, developmental stage and chemical treatment, BioMed CentralMolecular Biology, 9: 102
- nNaiche, L.A., Harrelson, Z., Kelly, R.G., Papaioannou, V.E., (2005). T-box genes in vertebrate development, Annual Review of Genetics, 39: 219-239.doi: 10.1146/annurev.genet.39.073003.105925
- nOwens, W., Koeter, H.B., (2003). The OECD program to validate the rat uterotrophic bioassay: an overview, Environmental Health Perspectives, 111(12):1527-1529. doi: 10.1289/ehp.6413
- nPreuss, T.G., Gehrhardt, J., Schirmer, K., Coors A., Rubach M., Russ A., Jones P.D., Giesy J.P., Ratte. H.T. (2006). Nonylphenol isomers differ in estrogenic activity, Envi-ronmental Science and Technology, 40(16): 5147-5153.doi: 10.1021/es060709r
- n Preuss, T.G., Gurer-Orhan, H., Meerman, J., Ratte, H.T. (2010). Some nonylphenol isomers show antiestrogenic potency in the MVLN cell assay, Toxicol in Vitro, 24(1): 129-134.doi 10.1016/j.tiv.2009.08.017
- nRuss, A.S., Vinken, R., Schuphan, I., Schmidt, B., (2005). Synthesis of branched para-nonylphenol isomers: occurrence and quantification in two commercial mixtures. Chemosphere. Sep 2005;60(11): 1624-1635.doi: 10.1016/j.chemosphere.2005.02.046
- nSchefe, J.H., Lehmann, K.E., Buschmann, I.R., Unger, T., Funke-Kaiser, H., (2006). Quantitative real-time RT-PCR data analysis: current concepts and the novel "gene expression's CT difference" formula, Journal of Molecular Medicine, 84(11): 901-910. doi: 10.1007/s00109-006-0097-6
- n Scholz, S., Fischer, S., Gundel, U., Kuster, E., Luckenbach, T., Voelker, D., (2008). The zebrafish embryo model in environmental risk assessment--applications beyond acute toxicity testing, Environmental Science and Pollution Research, 15(5): 394-404. doi: 10.1007/s11356-008-0018-z
- n Sharma, V.K., Anquandah, G.A., Yngard, R.A., Kim, H., Fekete, J., Bouzek, K., Ray, A.K., Golovko D., (2009) Nonylphenol, octylphenol, and bisphenol-A in the aquatic environment: a review on occurrence, fate,and treatment, Journal of Environmental Science and Health, Part A. Toxic/Hazardous Substances and Environmental Engineering, 44(5):423-442.doi: 10.1080/10934520902719704
- n Stachel, B., Jantzen, E., Knoth, W., Krüger, F., Lepom, P., Reinicke, H., Sawal, G., Schwartz, Uhlig S. (2005). The Elbe flood in August 2002--organic contaminants in sediment samples taken after the flood event, Journal of Environmental Science and Health, 40(2): 265-287
- nSchulte-Merker, S., Hammerschmidt, M., Beuchle, D., Cho, K.W., De Robertis, E.M., Nusslein-Volhard, C., (1994). Expression of zebrafishgoosecoid and no tail gene products in wild-type and mutant no tail embryos, Development, 120(4): 843-852
- n Wardle, F.C., Papaioannou, V.E., (2008). Teasing out T-box targets in early mesoderm, Current Opinion in Genetics and Development, 18(5): 418-425. doi: 10.1016/j.gde.2008.07.017
- nWheeler, T.F., Heim, J.R., LaTorre, M.R., Janes, A.B., (1997). Mass spectral characterization of p-nonylphenol isomers using high-resolution capillary GC-MS, Journal of Chromatographic Science, 35(1): 19-30