Keywords
Hormones; Environmental; Sex differentiation; Anguilla anguilla
Introduction
European eel (Anguilla anguilla) is a teleost species having a complex lifecycle both in sea and freshwater environments (Tesch, 1977). High variation in growth was discovered in European eel grown under aqua cultural conditions (Degani and Levanon, 1983, Crivelli., 1994).
Under natural conditions, many biological aspects of European eel were studied, include lifecycle, spawning behavior, migration, growth and metamorphosis from larvae to mature. The sex ratio in silver eels varies considerably between localities, ranging between 0 to 100% for females (Tesch 1977, Tesch 1978 , Tesch 1980, Tesch 1982 , Tesch 1989, Tesch 2003). The lifecycle, evolution and reproduction of European eel were reviewed in detail (Ginneken 2005). Many aspects of eel growth and nutrition were described under artificial conditions, which have been reviewed and summarized (Degani and Gallagher, 1995) (Figure 1).

Figure 1: European eel grown under aquacultural conditions.
Significant differences between growth rate and size have been observed between the two sexes, with females growing faster and larger than males (Degani et al., 2003b). The environment has been found to affect growth and sex determination in eels; females continue to grow, while the males cease to do so. Some males weigh between 15-200 g. Many theoretical and applied aspects of European eel, including hormone involvement in growth and reproduction, have been investigated (Degani and Gallagher, 1995). The administration of various hormones in eel diets influences growth (Degani 1986, Degani and Dosoretz 1986, Degani and Gallagher 1985). In European eel, molecular cloning of the PACAP precursor has been previously shown in fish, PACAP and GH-releasing hormone (GHRH) originating from the same precursor. The effects of PACAP and GHRH on GH secretion from eel pituitary cells in primary culture have been described (Montero 1998). The growth hormone sequence analysis and transcription of European eel were studied (Degani et al., 2003b), as well as gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (Degani et al., 2003a). Japanese eel (Anguilla japonica) Kazeto et al., 2008, folliclestimulating hormone (FSH) and luteinizing hormone (LH) produce biologically active recombinant FSH and LH by Drosophila S2 cells and have differential actions on reproductive biology Aroua et al., 2012. Plasma progesterone and estradiol increase after heterologous gonadotropin (hCG) treatment Colombo et al., 1987.
The effect of hormones on sex determination and growth by steroids (Degani and Gallagher 1985, G.D., 1985, Degani and Kushnirov 1992a, Tzchori et al., 2004a) was studied in detail.
In this mini-review, a model is presented suggesting that the relationship between the environment, hormones and sex determination affects growth. The relevance of such a model, not only in providing basic information, but also with respect to aquaculture, is described.
Environmental effect on sex differentiation and growth of European eel
The European eel is a catadromous and carnivorous species having a complex lifecycle in sea and freshwater (Degani and Gallagher, 1995). The European eel spawn in the Sargasso Sea in late winter and spring (Tesch, 1977, Tesch, 1978). The leafshaped larvae of European eel (leptocephali) (Tesch, 1980) are brought to the continental shelf of Europe by the Gulf Stream.
Before the eel enter into the freshwater of rivers in Europe and Mediterranean countries, the larvae metamorphose to glass eels. During periods in freshwater, they undergo growth and maturation, and change their pigments to yellow eels and silvery eels. Sex differentiation in eels occurs during the yellow eel stage (Colombo and Grandi, 1990, Colombo G, 1987, Geffroy, 2015). The eels become sexually mature at the silver eel stage. Young eels live in freshwater, where they stay for a period of 6-12 years for males and 9-18 years for females (Tesch 1977). As silver eels become sexually mature, they swim back to the sea and spawning grounds in the Sargasso Sea (Tesch 1977). Eel is an antic animal that lives in solitary in the ground (Tesch 1977). Based on experimental growing of eel in artificial and aquacultural conditions, many data support the density effect on sex differentiation (Figure 1). European eels maintain high densities, with the percentage of males being significantly higher compared to females.
In artificial conditions during growth, a very high variation in growth was found (Degani and Levanon, 1983), and the important environmental factor affecting growth was density (Degani and Gallagher, 1995). The rapid-growth eels become females and the slow-growth eels become males (Degani and Kushnirov, 1992b). The high density of growth significantly reduces growth, and estradiol increases growth and the percentage of females in the population (Degani and Kushnirov, 1992b). The relationship between environment and sex differentiation among males and females is presented in Figure 2.
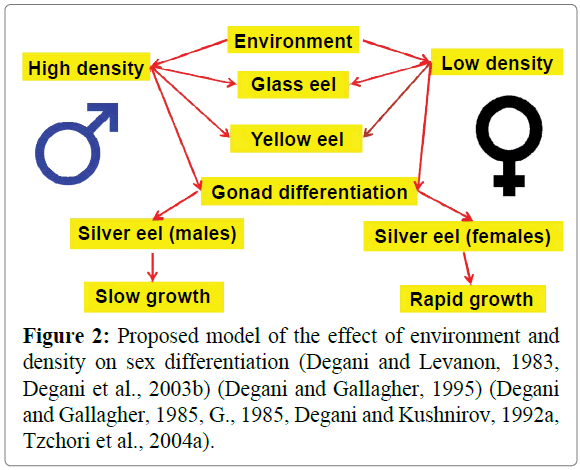
Figure 2: Proposed model of the effect of environment and density on sex differentiation (Degani and Levanon, 1983, Degani et al., 2003b) (Degani and Gallagher, 1995) (Degani and Gallagher, 1985, G., 1985, Degani and Kushnirov, 1992a, Tzchori et al., 2004a).
The proposed model described in Figure 1 might be explained by a study on hormone control reproduction and growth. In other words, the effect of the environment is on the hormonal axis.
Hormonal involvement in sex differentiation in eel
European eels belong to teleosts, as in other vertebrates, and growth and reproduction are tightly regulated, mainly via the hypothalamus-pituitary axis (Levy et al., 2009). The hypothalamic decapeptide, gonadotropin-releasing hormone (GnRH), controls the release of pituitary gonadotropins, folliclestimulating hormone (FSH) and luteinizing hormone (LH), which, in turn, control gametogenesis (Yaron et al., 2003). It is becoming increasingly evident that growth hormone (GH) plays a role in reproduction (Levy and Degani, 2013, Levy and Degani, 2012, Levy and Degani, 2011). A complex regulatory system involving multiple stimulatory and inhibitory factors may control the GH axis. In addition to its role in development and somatic growth, GH is involved in spermatogenesis regulation (Degani et al., 2003b).
Previous studies support the hypothesis that sex hormones affect growth in European eel. Moreover, the administration of 17β estradiol (E2) in the diets affects growth more than 17α-methyltestosterone (MT) (Degani, 1986). A high percentage (70%) of females was found among the European eels administered E2 (60 mg/Kg diet). This result is supported by other studies (Tzchori et al., 2004b, Tzchori et al., 2004a). The hormone controlling the E2 is FSH. Cloned (Aroua et al. 2012) cDNA of European eel and the mRNA level of FSH were significantly higher than in males (Degani et al., 2003a). Moreover, the expression of aromatase (EeCYP19) gene during the process of sex determination in adult male gonads was lower when compared to the adult female group (Tzchori et al., 2004b, Tzchori et al., 2004a).
On the other hand, the transcription of GH is significantly higher in female eels than in males (Degani et al., 2003b). In the model for the environmental effect on sex differentiation and growth (Figure 2), it is proposed that low density in the hypothalamuspituitary- gonad axis is affected by high FSH (Degani et al., 2003a) and gonad synthesis E2 by EeCYP19, and E2 affects gonad to ovary differentiation (Tzchori et al., 2004b, Tzchori et al., 2004a).
E2 affects the increase in growth compared to testosterone (Degani, 1986), and GH transcription in females was significantly higher than in males (Degani et al., 2003b). These results show the effect of E2 on GH in the model (Figure 2). Growth hormone is affected both directly by growth and the insulin-like growth factor (IGF) system in the liver. The administration if insulin in the European eel diet significant increases growth rate (Degani and Abraham, 1992). In Japanese eel (Anguilla japonica), insulinlike growth factor-II (IGF-II) cDNAs were cloned and their mRNA expression was examined in several tissues. Two eel IGFII cDNAs, eIGF-II-1 and eIGF-II-2, were cloned from the liver (Moriyama et al., 2008, Moriyama et al., 2000).
In high density conditions where European eel grow, the mRNA level of FSH was significantly lower in males (Degani et al., 2003a), and the inhibition to EeCYP19 significantly reduced the level of E2. Moreover, the expression of aromatase (EeCYP19) gene during the process of sex determination in adult male gonads was lower when compared to the adult female group (Tzchori et al., 2004b, Tzchori et al., 2004a). The no differentiation gonads became testis and secreted 11 KT, and low E2 had a lesser effect on growth than high E2 (Figure 3).
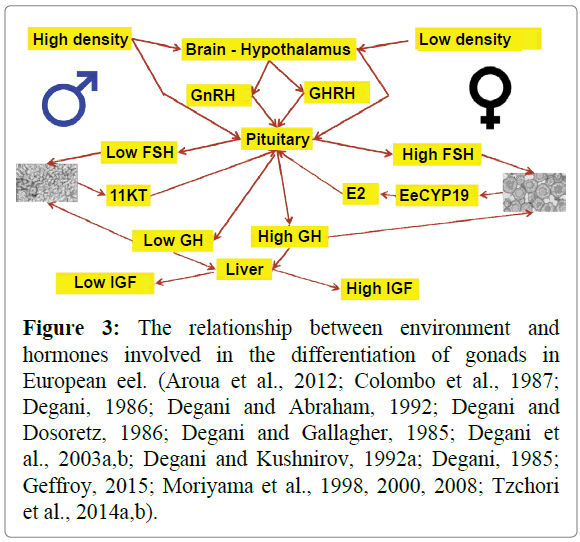
Figure 3: The relationship between environment and hormones involved in the differentiation of gonads in European eel. (Aroua et al., 2012; Colombo et al., 1987; Degani, 1986; Degani and Abraham, 1992; Degani and Dosoretz, 1986; Degani and Gallagher, 1985; Degani et al., 2003a,b; Degani and Kushnirov, 1992a; Degani, 1985; Geffroy, 2015; Moriyama et al., 1998, 2000, 2008; Tzchori et al., 2014a,b).
10578
References
- Tesch, F. (1977) The Eel Biology and Management of Anguillid Eel, Chapman and Hall, London
- nDegani, G., Levanon, D. (1983) The influence of low density on food adaptation, cannibalism, and growth of eels [Anguilla anguilla (L.)]. Isr J Aquacult Bamidgeh 35, 53-60
- nCrivelli., J.P.a.M.-C.X.a.A.J. (1994) Sources of Variation in Growth of the European Eel (Anguilla anguilla) Estimated from Otoliths Can. J. Fish. Aquat. Sci. 51
- nTesch, F.W. (1978 ) Telemetric observations on the spawning migration of the eel (Anguillaanguilla) west of the European continental shelf. . Env. Biol. Fish. 3,203-209
- nTesch, F.W. (1980) Occurrence of eel Anguilla anguilla larvae west of the Europeancontinental shelf, 1971-1977. Env. Biol. Fish. 5, 185-190
- nTesch, F.W. (1982 ) The Sargasso Sea Eel Expedition 1979. Helgoländer Meeresunsters 35, 263-277
- nTesch, F.W. (1989) Changes in swimming depth and direction of silver eels (Anguillaanguilla L.) from the continental shelf to the deep sea. . Aquat.Living Resour 2,9-20
- nGinneken, V.V. (2005) The European eel (Anguilla anguilla, Linnaeus), its Lifecycle, Evolution and Reproduction Fish Biology and Fisheries 15, 367-398
- nDegani, G., Gallagher, M.L. (1995) Growth and Nutrition of Eels, Laser Pages Publishing, Israel
- nDegani, G., Tzchori, I., Yom-Din, S., Goldberg, D., Jackson, K. (2003b) Growth differences and growth hormone expression in male and female European eels [Anguilla anguilla (L.)]. Gen Comp Endocrinol 134, 88-93
- nDegani, G. (1986) Effect of combined dietary 17βb estradiol and 17αa-methyltestosterone on growth and body composition of European eels (Anguilla anguilla). Aquaculture 59, 169-175
- nDegani, G., Dosoretz, C. (1986) The effect of 3,3',5-triiodo-L-thyronine and 17-alpha-methyltestosterone on growth and body composition of the glass stage of the eel (Anguilla anguilla L). Fish Physiol Biochem 1, 145-151
- nDegani, G., Gallagher, M.L. (1985) Effects of dietary 17-I-methyltestosterone and bovine growth hormone on growth and food conversion of slow and normally growing Amercan elvers (Anguilla rostrata). Can. J. Fish. Aquatic. Sci 42, 610-629
- nMontero M, Y.L., Rousseau, K., Arimura, A., Fournier, A., Dufour, S. et al. (1998 ) Distribution, characterization, and growth hormone-releasing activity of pituitary adenylate cyclase-activating polypeptide in the European eel, Anguilla anguilla. . Endocrinology 139, 4300-4310
- nDegani, G., Jackson, K., Goldberg, D., Sarfati, R., Avtalion, R.R. (2003a) betaFSH, betaLH and growth hormone gene expression in blue gourami (Trichogaster trichopterus, Pallas 1770) during spermatogenesis and male sexual behavior. Zoolog Sci. 20, 737-743
- nKazeto, Y., Kohara, M., Miura, T., Miura, C., Yamaguchi, S. et al. (2008) Japanese Eel Follicle-Stimulating Hormone (Fsh) and Luteinizing Hormone (Lh): Production of Biologically Active Recombinant Fsh and Lh by Drosophila S2 Cells and Their Differential Actions on the Reproductive Biology. Biology of reproduction 79, 938-946
- nAroua S., M.G., Jeng S.R., Chang C.F., Weltzien F.A., Rousseau K. et al. (2012) Pituitary gonadotropins FSH and LH are oppositely regulated by the activin/follistatin system in a basal teleost, the eel. Gen Comp Endocrinol 175,82-91
- nColombo G, G.G., Romeo, A., Giovannini, G., Pelizzola, D., Catozzi, L. et al. (1987) Testis cytological structure, plasma sex steroids, and gonad cytosol free steroid receptors of heterologous gonadotropin (hCG)-stimulated silver eel, Anguilla anguilla L. Gen Comp Endocrinol 65, 167-178
- nDegani, G.(1985) The influence of17b-methyltestosterone on body composition of eels (Anguilla anguilla). Aquaculture 50, 23-30
- nDegani, G., Kushnirov, D. (1992a) Effects of 17a-estradiol and grouping on gender determination of European eels. Prog. Fish Cult 54, 88-91
- nTzchori, I., Degani, G., Elisha, R., et al. (2004a) The influence of phytoestrogens and oestradiol-17β on growth and sex determination in the European eel (Anguilla anguilla) Aquaculture Research 35, 1213-1219
- nColombo, G., Grandi, G. (1990) Gonad sex differentiation of Anguilla anguilla by sex steroid. International Reviews in Hydrobiology 75, 763-773
- nGeffroy, B.B. A. (2015) Sex differentiation and sex determination in eels: consequences for management FISH and FISHERIES John Wiley and Sons ltd 1-24
- nDegani, G., Kushnirov, D. (1992b) Effects of 17a-estradiol and grouping on sex determination of European eels. Prog Fish Cult 54, 88-91
- nLevy, G., Gothilf, Y., Degani, G. (2009) Brain gonadotropin releasing hormone3 expression variation during oogenesis and sexual behavior and its effect on pituitary hormonal expression in the blue gourami. Comp Biochem Physiol A Mol Integr Physiol 154, 241-248
- nYaron, Z., Gur, G., Melamed, P., Rosenfeld, H., Elizur, A. et al. (2003) Regulation of fish gonadotropins. Int Rev Cytol 225, 131-185
- nLevy, G., Degani, G. (2012) Involvement of GnRH, PACAP and PRP in the reproduction of blue gourami females (Trichogaster trichopterus). J Mol Neurosci 48, 603-616
- nLevy, G., Degani, G. (2011) Evidence of a reproduction-related function for pituitary adenylate cyclase-activating polypeptide-related peptide in an Anabantidae fish. J Mol Endocrinol 46, 101-110
- nTzchori, I., Degani, G., Hurvitz, A., Moav, B. (2004b) Cloning and developmental expression of the cytochrome P450 aromatase gene (CYP19) in the European eel (Anguilla anguilla). Gen Comp Endocrinol 138, 271-280
- nDegani, G., Abraham, M. (1992) Effect of insulin in the diet on the growth of European eels (Anguilla anguilla (L.). Fish Physiol Biochem 10, 223-227
- nMoriyama, S., Yamaguchi, K., Takasawa, T., Chiba, H., Kawauchi, H. ( 2008) Identification of two insulin-like growth factor IIs in the Japanese eel, Anguilla japonica: cloning, tissue distribution, and expression after growth hormone treatment and seawater acclimation.Comp Biochem Physiol B Biochem Mol Biol.149, 47-57
- nMoriyama, S., Ayson, F.G., Kawauchi, H. (2000) Growth regulation by insulin-like growth factor-I in fish. Biosci Biotechnol Biochem64, 1553-1562.









