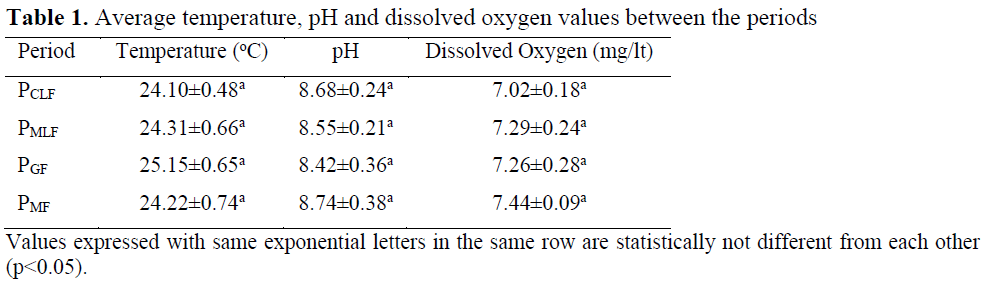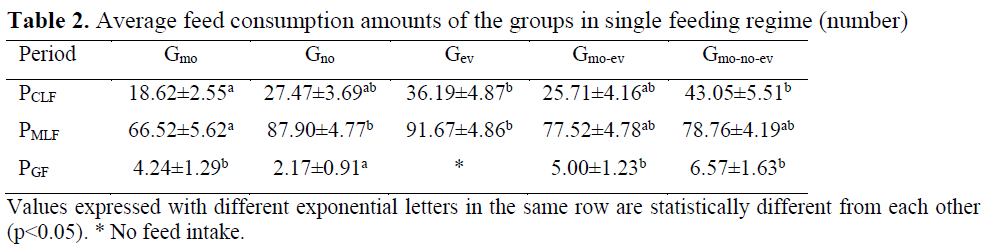Keywords
Flounder (Platichthys flesus), Live food, Feed consumption, Food preference
Introduction
Flounder (Platichthys flesus) is a species of Pleuronectidae order, which is spread in the less salty waters of Western Europe from White Sea to the Mediterranean and Black Sea (Nielsen, 1986). The species is a flat fish species of economic value for Black Sea (Aarnio et al., 1996; Ayd?n, 2012). Studies on breeding of flounder started in the 1960s in Japan and in 1970s in England and France, but facussed mainly the solve and studies for breeding turbot (Psetta maxima) after 1997 (Matsuoka, 1996; Yi?it, 2001).
The studies with flounder in Turkey included trials on transfer, from the wild to culture works adaptation and feeding, breeding requirements, juvenile production, reproduction characteristics, egg development, revealing of exterior abnormalities seen in reproduction performance and hatchery production (Ergün & Yalç?n, 2006; Ayd?n et al., 2011; Ayd?n, 2012). In aquaculture, larvae of especially several fish species require live food. Knowing feeding habits and food preferences of interested fish species is important especially for growth and survival of juvenile fish (Aarnio, 2000; Ghosh et al., 2003).
The study seeks to determine feed prefer and consumption amounts of the flounder based on use of different feed options such as chironomid larvae, mosquito larvae and pelleted feed.
Materials and Methods
The fish were captured in June (2010) by seines and trawl nets in the estuarine zone where S?rakaraa?açlar Creek flows into the sea at 10 km west of Sinop province. The fish were brought alive to Sinop University, Aquaculture Faculty Research Center, and stocked in adaptation tanks for 15 days.
15 fish with an average weight of 0.48±0.03 g and average length of 36.11±0.77 mm were stocked individually in the system composed of plastic containers of 10 lt. with continuous aeration. Three replications in order to determine individual consumption of the fish was placed in each group only one fish. Chironomid (Chironomous sp.) and mosquito larvae (Culex sp.) was obtained from the water channel coming from the research center. Chironomid larvae (phase 4; 10-12 mm), mosquito larvae (phase 4, instar IV; 6-7 mm) and pellet feed (0.8-1 mm) were used for nutrition. The dimension of the prey according to the dimensions of the mouth opening of the fish was determined.
The study was conducted three repeated as in five groups of three different feeding times [Morning (Gmo), Noon (Gno), Evening (Gev), Morning-Evening (Gmo-ev), Morning-Noon- Evening (Gmo-no-ev)] under natural lighting regime. In order to standardize the fasting state in the fish, feed was not given for 24 hours following placing in the trial tanks. The feed were given singly [120 chironomid larvae (PCLF), 120 mosquito larvae (PMLF) and 120 pellet feed (PGF)] and as a mixture [120 chironomid larvae + 120 mosquito larvae + 120 pellet feed (PMF)] for 7 days.
Feeding took place at 0900 in the morning, at 1300 at noon and at 1700 in the evening. After 3 hours from feeding, the feed were counted and collected, consumed feed amounts were calculated and average feed consumption amounts (±SD) were determined (Ghosh et al., 2003). Temperature, pH and dissolved oxygen values were measured in-situ three times a day with a WTW branded device. In the study, the difference between the groups was determined by one-way anova analysis and significance of the difference was determined by Duncan’s multiple comparison test. IBM SPSS 21.0 programme was used in the calculations.
Results and Discussion
No difference was seen in temperature, pH and dissolved oxygen values (p>0.05) (Table 1).
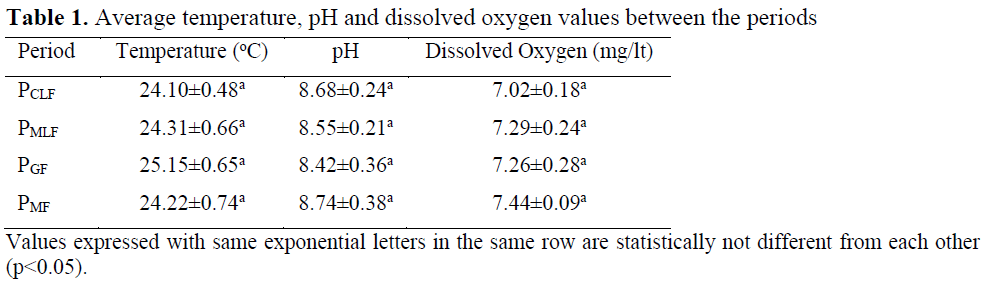
Table 1. Average temperature, pH and dissolved oxygen values between the periods
Death was not observed in the groups during the study. Food consumptions of the groups in single and mixed feeding regimes are given in Table 2 and Table 3.
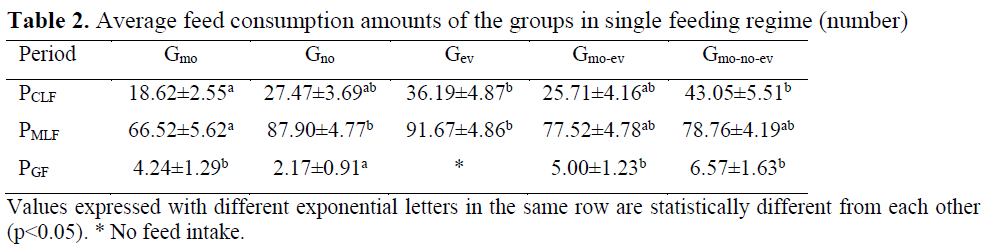
Table 2. Average feed consumption amounts of the groups in single feeding regime (number)
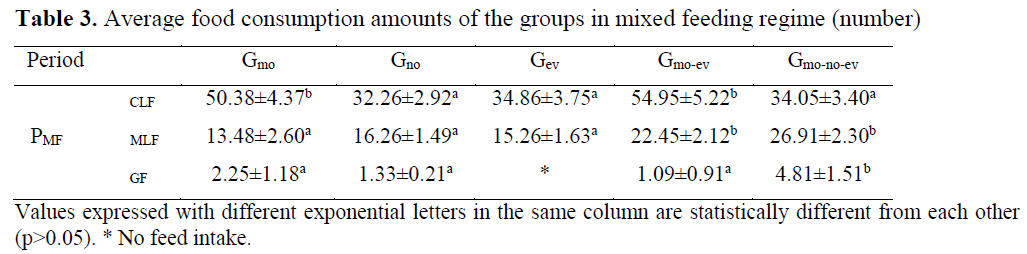
Table 3. Average food consumption amounts of the groups in mixed feeding regime (number)
There was irregularity between the days in chironomid consumption in Gmo and Gno groups and there was regular feed intake in Gev, Gmo-ev and Gmo-no-ev groups. As a result of the analysis conducted between the groups on daily chronomid larvae consumption amounts, the difference between the groups Gmo and Gev and Gmo-no-ev was significant (p<0.05). Consumption of chironomid larvae was higher particularly in the evening meals compared to other meals. Moreover, consumption of chironomid larvae in aggregate was the highest in Gmo-no-ev group and the lowest in Gmo group.
Mosquito larvae consumption was regular in all groups. In Gmo, Gno and Gev groups, mosquito larvae consumption for the first three days was lower compared to the other days whereas in Gmo-ev and Gmo-no-ev groups, mosquito larvae consumption increased regularly. As a result of the statistics conducted between the groups on daily mosquito larvae consumption amounts, the difference between the groups Gmo and Gno and Gev was e significant (p<0.05). Average mosquito consumption amount during the feeding period was the highest in Gev group and the lowest in Gmo group.
In feeding with pellet feed, no consumption was observed in Gev group whereas consumption in other groups was irregular. In this feeding period in which individual preferences became prominent due to failure to adapt to granule feed, the difference in daily granule feed consumption amounts between Gno group and other groups was significant (p<0.05).
In mixed feeding, chironomid larvae consumption increased in Gmo and Gmo-ev groups (p<0.05), mosquito larvae consumption decreased in all groups (p<0.05), and in feeding with granule feed, the difference between consumption amounts was insignificant (p>0.05).
While studies of adaptation to the feed having different ration characteristics with different live food options in feeding were conducted, very little information is available on their feeding especially in lagoons with hard water character since flounder is a species that is characterized as an alternative species.
Feed intake of the fish varies depending on its hunting character, quality, density, physical attractiveness and length of the feed. In several studies, it is reported that some fish preferred mosquito larvae due to behavioral characteristics of the feed (Kumar et al., 2008; Devi & Jauhari, 2011). Flounder shows a character of staying steady in natural environment but making sudden movement towards the feed during hunting (Yi?it, 2001). It is reported that artificial food and other alternative food sources are used at the time when feed intake from outside starts in larva culture of several fish species (Legendre et al., 1995). Furthermore, it is stated that granule feed prepared so as to satisfy feed requirements of fish larvae and juveniles could not be utilized due to non-development of adaptation to the granule feed or problems suffered with respect to digestion of the feed (Kowen et al., 2001). Ergün and Yalç?n (2006) reported that flounder did not prefer granule feed initially; therefore, mortality rate was high.
In the nature, chironomid larvae are motile on the ground whereas mosquito larvae have limited motility on the surface (Titmus & Bodcock, 1981; Real et al., 2000; Bat & Akbulut, 2001; Özkan, 2006). Mosquito larvae are utilized in feeding of various fish species for use in biologic struggle (Fletcher et al., 1993; Lardeux et al., 2002; Y?ld?r?m & Karaçuha, 2007).
Benthic invertebrates take an important part in feeding of juvenile fish that live in the bottom during the entire of or several periods of their lifespan (Fischer & Eckmann, 1997). Chironomid larvae are reported to be a feed source that lives in lower substrates, has an important potential of live food, is preferred for its wriggling movements, has a high and rich nutrition content (55.7% raw protein and 9.7% fat) and is easy to digest for juvenile fish (Steffens, 1960; James et al., 1993; Branco et al., 1997; Bogut et al., 2007; Ferrington et al., 2008).
Mattila & Bonsdorff (1998), conducted a study to determine the predator behavior of the flounder on Macoma balthica and Bathyporeia pilosa species, stated that giving the two feed separately or in combination did not make difference on consumption amount, but increases in the total feed amount by increased consumption amount.
In breeding of bullhead (Silurus glanis) (Ronyai and Ruttkay, 1990), juvenile burbot (Lota lota) (Hofmann & Fischer, 2003) and channel bullhead (Ictalurus punctatus) larvae (Mulligan et al., 2010), chironomid larvae are one of the important live food sources and can be used as live food. Adamek et al. (2007) reported that sturgeon (Acipenser baerii) larvae preferred chironomid larvae, and could be utilized in alternative feed rations.
Manna et al. (2008) stated that guppy (Poecilia reticulata) preferred chironomid and tubifex larvae as live food. Gupta & Banerjee (2009) reported that goldfish (Carassius auratus) showed high preference towards live food compared to artificial feed, and the most preferred one among the live food was chironomid larvae.
In the lagoons which are among the natural habitats of flounder, it was reported that a substantial part of the benthic macro invertebrates consisted of chironomid larvae (Çamur-Elipek et al., 2010; Özkan et al., 2010). Preferences of juvenile flounder were copepod up to 45 mm, and oligochaeta, amphipoda and chironomid between 45 and 101 mm (Aarnio et al., 1996). Flounder mostly fed with benthic organisms (Aarnio, 2000). Nissling et al. (2007) reported that juvenile flounder over 40 mm mostly preferred oligochaeta and chironomidae species.
Conclusion
In the present study, chironomid and mosquito larvae, which are utilized as feed source by the fish in the nature, and commercially produced granule feed were used and consumption amounts and food preferences were tried to be determined based on number of meals and meal times of the flounder. The obtained data suggest that flounder preferred chironomid larvae more compared to mosquito larvae and granule feed. In all groups, chironomid larvae and mosquito larvae were notably preferred whereas in mixed feeding chironomid larvae consumption increased and mosquito larvae consumption decreased. The fish significantly prefer the chironomid larvae that has the feature of motility on the ground at every meal.
The studies conducted that chironomid larvae, which are rapidly digested and reported to be a good protein source (Özkan, 2006), are one of live food sources preferred for their motile behavior in the water and easily available for demersal species such as flounder, and can be utilized in alternative feed rations.
6448
References
- nAarnio, K., (2000). Experimental evidence of predation by juvenile flounder, Platichthys flesus, on a shallow water meiobenthic community, Journal of Experimental Marine Biology and Ecology, 246(1): 125-n138. doi: 10.1016/S0022-0981(99)00175-n6
- nAarnio, K., Bonsdorff, E., Rosenback, N., (1996). Food and feeding habits of juveline flounder Platichthys flesus (L.), and turbot Scophthalmus maximus L. in the aland archipelago, northern Baltic Sea, Journal of Sea Research, 36(3-4): 311-320. doi: 10.1016/S1385-1101(96)90798-4
- nAdamek, Z., Prokes, M., Barus, V., Sukop, I., (2007). Diet and growth of 1+Siberian Sturgeon, Acipenser baerii in alternative pond culture, Turkish Journal of Fisheries and Aquatic Sciences, 7(2): 153-160.
- nAydın, İ., (2012). The external abnormalities of hatchery-reared Black Sea flounder (Pleuronectes flesus luscus, Pallas, 1814) in Turkey, Turkish Journal of Fisheries and Aquatic Sciences, 12(1): 123-128.ndoi: 10.4194/1303-2712-v12_1_15.
- nAydın, İ., Şahin, T., Polat, H., Güneş, E., (2011). The reproductive performance of wild and hatchery-reared flounder, Platichthys flesus luscus, in the southern Black Sea coast, Turkish Journal ofnZoology, 35(6): 811-817. doi: 10.3906/zoo-1004-1.
- nBat, L., Akbulut, M., (2001). Studies on sediment toxicity bioassays using Chironomous thumni (K., 1991) larvae. Turkish Journal of Zoology, 25: 87-93
- nBogut, I., Has-Schon, E., Adamek, Z., Rohkovic, V., Galovic, D., (2007). Chironomus plumosus larvae-a suitable nutrient of freshwater farmed fish, Agricultural Scientific and ProfessionnReview, 13(1): 159-162
- nBranco, C.W.C., Aguiaro, T., Esteves, F.A.,nCaramaschi, E.P., (1997). Food sources of the Teleost Eucinostomus argenteus in twoncoastal lagoons of Brazil, Studies onnNeotropical Fauna and Environment,n32(1): 33-40. doi:n10.1076/snfe.32.1.33.13463
- nÇamur-Elipek, B., Arslan, N., Kirgiz, T., Öterler, B., Güher, H., Özkan, N., (2010). Analysis of benthic macroinvertebrates in relation to environmental variables of Lake Gala, a National Park of Turkey, Turkish Journal of Fisheries and Aquatic Sciences,n10(2): 235-243. doi:n10.4194/trjfas.2010.0212
- nDevi, N.P., Jauhari, R.K., (2011). Food preference of Aplocheilus panchax (Cyprinidontiformes: Aplocheilidae) with special reference towards, Researcher, 3(6): 55-59,
- nErgün, S., Yalçın, M., (2006). A study on thenadaptation and feeding of wild-caught flounder (Platichthys flesus) juveniles in aquaculture conditions. Ege UniversitynJournal of Fisheries & Aquatic Sciences,n23(1/2): 215-218
- nFerrington, L.C,, Jr. Berg, M.B., Coffman,nW.P., (2008). Chironomidae. In: AnnIntroduction to the Aquatic Insects ofnNorth America (ed. by R.W. Merritt, K.W.nCummins & M.B. Berg), 4th edn, pp. 847-n989,
- nKendall/Hunt, Dubuque, IA, USA.nFischer, P., Eckmann, R., (1997). Seasonal changes in fish abundance, biomass and species richness in the littoral zone of anlarge European lake, Lake Constance, Germany, Archiv für Hydrobiologie, 139:n433–448
- nFletcher, M.A., Teklchaimanot, A., Yeman,e G., Kassahum, A., Kidane, G., Beyene, Y., (1993). Prospects for the use of larvivorous fish for malaria control in Ethiopia: search for indigenous species and evaluation ofntheir feeding capacity for mosquitonlarvae, The American Journal of TropicalnMedicine and Hygiene96(1): 12-21
- nGhosh, A., Mahapatra, B.K., Data, N.C., (2003). Ornamental fish farmingsuccessful small, Scale Aqua Businessin India, Aquaculture Asia, VIII(3):14-16.nGupta, S., Banerjee, S., (2009). Food preference of goldfish (Carassius auratus) and its patential in mosquito control, ElectronicnJournal of Ichthyology, 5(2): 47-58
- nHofmann, N., Fischer, P., (2003). Impact of temperature on food intake and growth in juvenile burbot, Journal of Fish Biology,n63(5): 1295–1305. doi: 10.1046/j.1095-n8649.2003.00252.x
- nJames, R., Muthukrishnan, J., Sampath, K., (1993). Effects of food quality on temporal and energetics cost of feeding innCyprinus carpio (Cyprinidae), J o u r n a lno f Aquaculture in the Tropics 8:n47–53
- nKowen, W., Barr, Y., Lutsky, S., Ben-Atia, I.,nWeiss, R., Harel, M., Behrens, P., Tandler, A., (2001). The effect of dietary arachidonic acid (20:4n-6) on growth, survival and resistance to handling stress in gilthead seabream (Sparus aurata) larvae,nAquaculture, 193(1-2): 107–122. doi: 10.1016/S0044-8486(00)00479-8.
- nKumar, D., Marimuthu, K., Haniffa, M.A,,nSethuramalingam, T.A., (2008). Effect ofndifferent live feed on growth and survival of Striped Murrel Channa striatus larvae,nEge University Journal of Fisheries, 25(2): 105–110
- nLardeux, F., Sechan, Y., Loncke, S., Deparis, X., Cheffort, J., Faaruia, M., (2002). Integrated control of peridomestic larval habitats of Aedes and Culex mosquitoes (Diptera: Culicidae) in atoll villages of French Polynesia, Journal of Medical Entomology, 39(3): 493-498. doi: 10.1603/0022-2585-39.3.493
- nLegendre, M., Kerdchuen, N., Corraze, G., Bergot, P., (1995). Larval rearing of an African catfish Heterobranchus longifilis (Teleostei, Clariidae): effect of dietary lipids on growth, survival and fatty acidncomposition of fry, Aquatic Living Resour,n8: 355-363. doi: 10.1051/alr:1995040
- nManna, B., Aditya, G., Banerjee, S., (2008). Vulnerability of the mosquito larvae to the guppies (Poecilia reticulata) in the presence of alternative preys, Journal Vector Borne Diseases, 45(3): 200-206
- nMatsuoka, T., (1996). Present state and prospects of Japan’s Sea farming, The International Symposium on Marine Ranching in Ishikawa, 1p. Kanazawa, Ishikawa Prefecture. Mattila, J., Bonsdorff, E., (1998). Predation by juvenile flounder (Platichthys flesus L.): antest of prey vulnerability, predator preference, switching behaviour andnfunctional pesponse, Journal of Experimental Marine Biology and Ecology, 227: 221-236. doi: 10.1016/S0022-0981(97)00272-4
- nMulligan, B.L., Morris, J.E., Clayton, R.D., (2010). Chironomid abundance and consumption by juvenile channel catfish in plastic-lined and earthen culture ponds, Aquaculture Research, 41: 234-238.ndoi: 10.1111/j.1365-2109.2010.02509.x
- nNielsen, J.G., (1986). Pleuronectidae. pp. 1299-1307. In: P.J.P. Whitehead, M.L. Bauchot, J.C.P. Nielsen, E. Tortonese (eds.). Fishes of the North-Eastern Atlantic and the Mediterranean, 3, Paris. 1302 p
- nNissling, A., Jacobsson, M., Hallberg, N.,n(2007). Feeding ecology of juvenile turbot Scophthalmus maximus and flounder Pleuronectes flesus at Gotland, Central Baltic Sea, Journal of Fish Biology, 70:n1877–1897. doi: 10.1111/j.1095-n8649.2007.01463.x
- nÖzkan, N., (2006). The larval chironomidae (Diptera) fauna of Bozcaada (Tenedos), Gazi University Journal of Science, 19(2):n57-67. Özkan, N., Moubayed-Breil, J., Çamur-Elipek,nB., (2010). Ecological Analysis of Chironomid Larvae (Diptera, Chironomidae) in Ergene River Basinn(Turkish Thrace), Turkish Journal of Fisheries and Aquatic Sciences, 10(1): 93-n99. doi: 10.4194/trjfas.2010.0114
- nReal, M., Rieradevall, M., Prat, N., (2000).nChironomous species (Diptera: Chironomidae) in the profundal benthos of Spanish reservoirs and lakes: factors affecting distribution patterns, Freshwater Biology, 43(1): 1-18. doi: 10.1046/j.1365-n2427.2000.00508.x
- nRonyai, A., Ruttkay, A., (1990). Growth and food utilization of wels fry (Silurus glanis) fed with tubifex, AquaculturenHung. (Szarvas) VI: 193-202
- nSteffens, W., (1960). Ernahrung und Wachstum des jungen Zanders (Lucioperca lucioperca) in Teichen, ZnFisch N.F., 9: 161–271
- nTitmus, G., Badcock, R.M., (1981).nDistribution and feeding of larval chironomidae in a gravel-pit lake, Freshwater Biology, 11(3): 263-271.ndoi: 10.1111/j.1365-2427.1981.tb01259.x.
- nYıldırım, Ö., Karaçuha, A., (2007). A preliminary study on determination ofnAphanius chantrei’s feeding behaviour on mosquito larvae, Acta Tropica, 102(3):n172-175.ndoi: 10.1016/j.actatropica.2007.04.016
- nYiğit, M., (2001). Different Protein and EnergynRates Japanese Flounder (Paralichtysnolivaceus) Juvenile Development, fish feeds of the chemical structure of his body, the effects of the rate of Nitrogen Disposal, 72p. Ph.D. thesis, Ondokuz Mayis University, Samsun, Turkey.