Key words
Cyprinids, Fecundity, Spawning time
Introduction
Tench, Tinca tinca (L., 1758), is one of the most abundant freshwater cyprinids in Europe and Asia, and is found in a variety of inland wa-ters throughout Turkey (Bal?k and Çubuk, 2000; Bal?k et al., 2004; Ergönül and Alt?nda?, 2005). Despite the country’s dependence on large lakes as an important economic resource, and the con-tinued construction of dams for increased hy-droelectric power and irrigation potential, very little is known about the fish populations in most lakes, or how these populations might respond to this ever increasing pressure of development.
From an economic perspective, tench is farmed throughout much of Europe (Wedekind et al., 2003), but until recently, was not a significant part of the aquaculture industry in Turkey (Bal?k et al., 2004). Tench aquaculture is mostly neg-ligible in Turkey despite its popularity in sport fishing (Alt?nda? et al., 2002) and its low sus-ceptibility to disease compared to other fish spe-cies (Svobodova and Kolarova, 2004). From an ecological perspective, tench have been intro-duced into many Turkish lakes in recent years, and catches have gradually increased (Bal?k and Çubuk, 2000). Tench are nocturnal, benthic pre-dators of various invertebrate grazers such as ga-stropod, molluscs, and are known to positively affect periphyton biomass in eutrophic lakes (Herrero et al., 2005; Beklio?lu and Moss, 1998; Adámek et al., 2003).
A few studies have characterized tench repro-duction in various inland water bodies throughout Turkey (Y?lmaz, 2002; Ala? and Solak, 2004; Benzer et al., 2007). The present study is impor-tant in terms of comparison of the results of Benzer et al. (2007) in Hirfanl? Dam Lake during 1996 - 1997. The primary aim of this study is to describe the tench population from Hirfanl? Dam Lake and correlate populations characteristics such as spawning period and fecundity.
Materials and Methods
Hirfanl? Dam Lake is located on the K?z?l?rmak River, 70 km south of K?r?kkale province in the Central Anatolia region of Turkey (Figure 1). The altitude, mean depth and reservoir area of the lake are 856 m, 83 m and 320 km2, respectively (DSI, 1973).
Figure 1. Map of Hirfanl? Dam Lake
Specimens were captured monthly by gill nets of various mesh sizes (18x18 mm, 40x40 mm, 55x55 mm) between June 2001 and May 2002. The fork length (FL) and weight (W) were meas-ured to the nearest 1.0 mm and 0.1 g, respec-tively. Ten to fifteen scales above the lateral line, below the anterior part of the dorsal fin were re-moved for age determination. Scales were waited in 4% potassium hydroxide solution for 24 hours and then washed and cleaned with a thin brush. Clean scales were left in 70% alcohol for 5-10 minutes and then mounted dry and bounded tightly between two slides with sellotape (Lagler, 1956). Sex and stage of maturity were ascer-tained by examination of the gonad tissue either by eye for bigger fish or with the aid of a micro-scope for smaller ones. The sexual maturity of the gonads was classified as: Stage 1, young in-dividuals which have never spawned yet; stage 2, gonads are of very small size and eggs not visible to the naked eye; stage 3, ripening; eggs visible to the naked eye; stage 4, ripeness; gonads have reached their maximum weight; stage 5, repro-duction; gametes run out on the application of the lightest pressure to the thorax and the weight of the gonad rapidly decreases from start to finish of the spawning process; stage 6, spent; gonad has the appearance of an empty sac, usually with a few eggs remaining in females or sperms in males (Nikolsky, 1963). Gonads were removed and weighed to the nearest 0.01 g and preserved in 4% formaldehyde. Sexual maturity and spawning period were determined from monthly variations of the gonadosomatic index (GSI) and egg diameters of samples (Nikolsky, 1963). GSI was calculated monthly from the equation GSI= (Wg/(Wt–Wg)x100 where Wg and Wt are gonad weight and total body weight in grams of fish, respectively. To estimate the mean lengths at 50% maturity, a logistic function was fitted to the proportion of the mature individuals by size class using a non-linear regression. The function used was P= 1/ [1+exp(-r(L-Lm))], where P is the pro-portion mature in each size class, r (-b slope) is a parameter controlling the shape of the curve and Lm is the size at 50% maturity. Lm = a/r, where a is intercept (Saila et al., 1988).
Fecundity (F) was estimated by the gravime-tric method (Bagenal and Braum, 1978): ovaries were washed for two hours, and three subsamples weighing 1 g each were taken from the front, middle and back parts of each ovary. The eggs in the subsamples were counted. The total number of eggs in each ovary was proportional to the to-tal ovary weight. Relative fecundity was calcu-lated as Fr= F/W (g) and Fr = F/FL (mm). Fecun-dity (F) – fork length (FL), fecundity – weight (W), fecundity – gonad weight (GW) and fecun-dity – age (t) relationships were determined from the equation F= a*xb where F= fecundity, x= length, weight, gonad weight or age, a= a con-stant and b= an exponent. All data was trans-formed to a logarithmic form as logF= loga + b logx (Bagenal and Braum, 1978). The diameters of 30 eggs taken from the front, middle and back parts of the mature ovaries were measured using a micrometer. The overall ratio of males to fe-males was tested with X2-test (Sümbülo?lu and Sümbülo?lu, 1994).
Results and Discussion
Sex ratio, age distribution and length fre-quency
Of the total fish examined, 116 were males (47.93%) and 126 were females (52.07%). The sex ratio of males to females was 0.92:1.00 and was not significantly different from 1.00:1.00 (X2= 0.41< X21, 0.05= 3.84). The differences be-tween the sexes according to age were significant in age groups III and V (p<0.05) (Table 1).
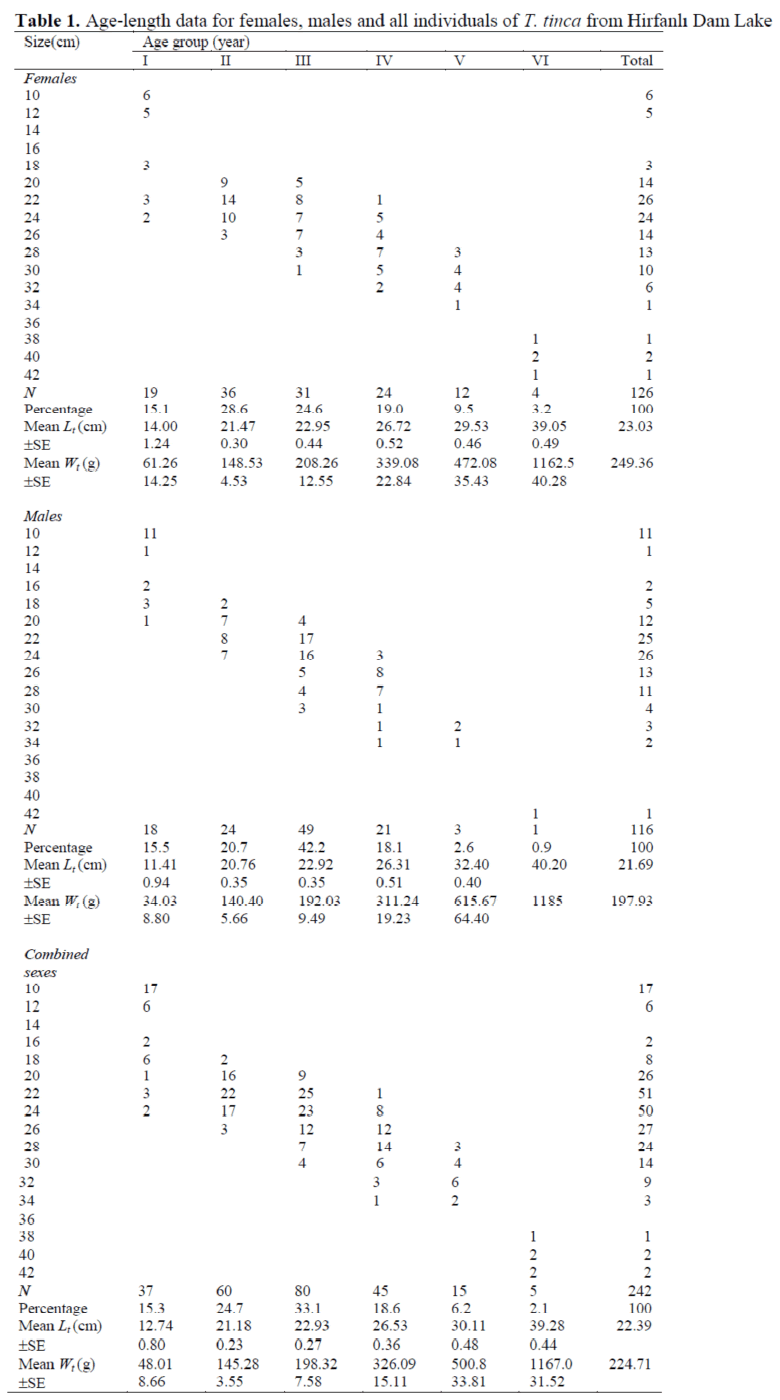
Table 1. Age-length data for females, males and all individuals of T. tinca from Hirfanl? Dam Lake
Females in all age groups were dominant in the population except in the third age. The ages of fish ranged from I to VI years and the third age group was dominant in the population (33.1%). The sampled fish ranged from 7.9 cm to 40.2 cm FL. The majority group (63.6%) belonged to 20-26 cm FL (Table 1).
Age and length at first maturity
The smallest mature female captured was 22 cm FL (250 g total weight) and the smallest ma-ture male was 19.6 cm FL (119 g total weight). First spawning age was IV for females and III for males. The maturity ogives for tench showed that 50% of females P= 1/ [1+ exp (-0.995(L-24))] and males P= 1/ [1+ exp (-0.972(L-22))] were sexually mature at fork lengths of 24 and 22 cm, respec-tively (Figure 2).
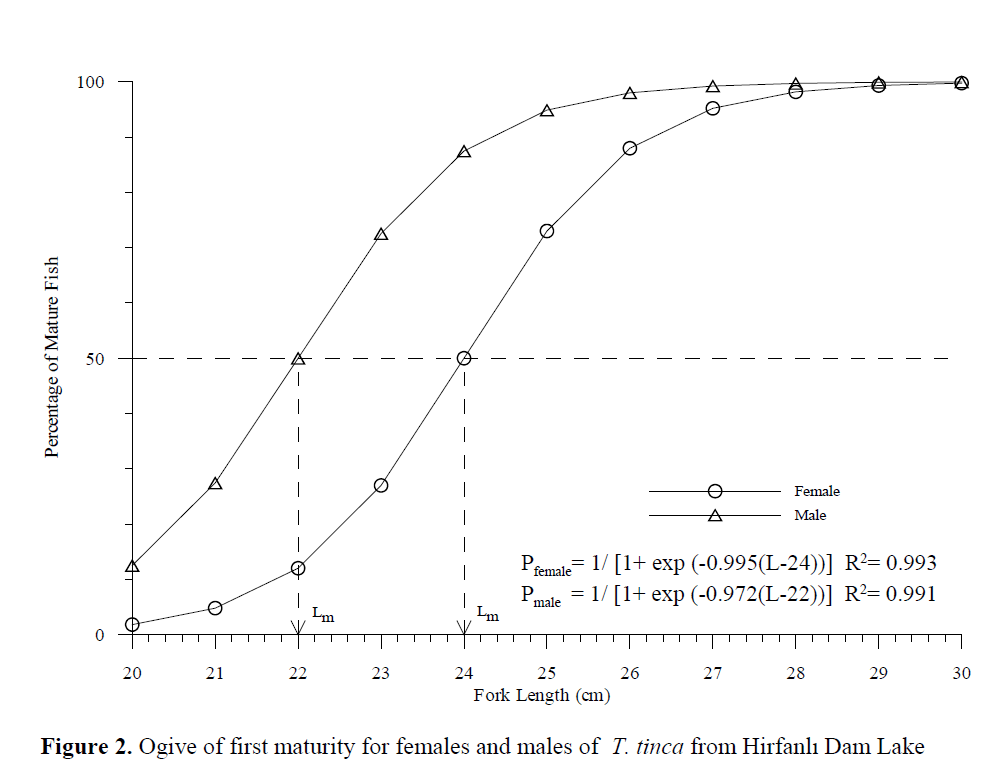
Figure 2. Ogive of first maturity for females and males of T. tinca from Hirfanl? Dam Lake
Gonad development and spawning period
Spawning occurred from the end of May (19ºC, GSI= 9.56) to the mid of July (25.8ºC, GSI= 4.35). After spawning, GSI values were almost steady until February. GSI increased drastically from February (2.16) to May (9.56) (Figure 3). Similarly, egg diameters of mature females increased rapidly from February to May. The mean egg diameter was the smallest in July (0.22 mm) and the highest in May (1.08 mm) (Figure 4). Six different gonad maturity stages were determined for females. The monthly distri-bution of the maturity stages in the period from June 2001 to May 2002 is shown in Figure 5.
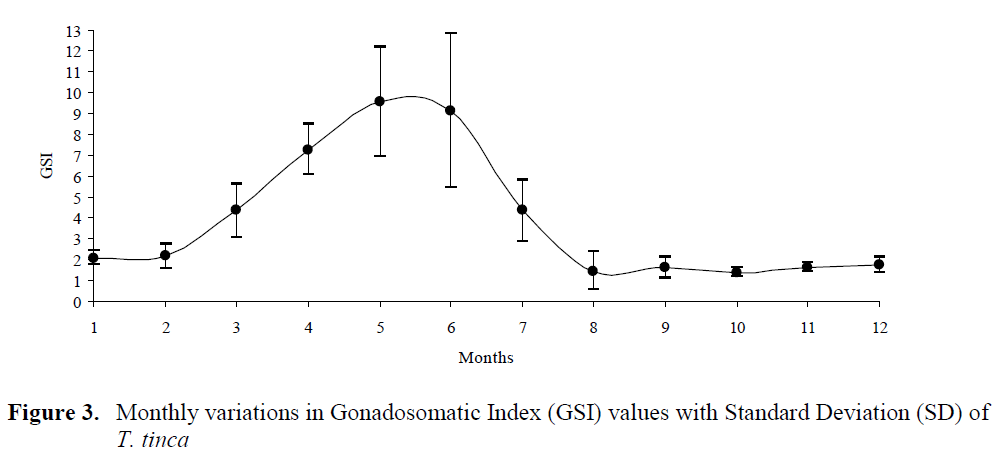
Figure 3. Monthly variations in Gonadosomatic Index (GSI) values with Standard Deviation (SD) of T. tinca
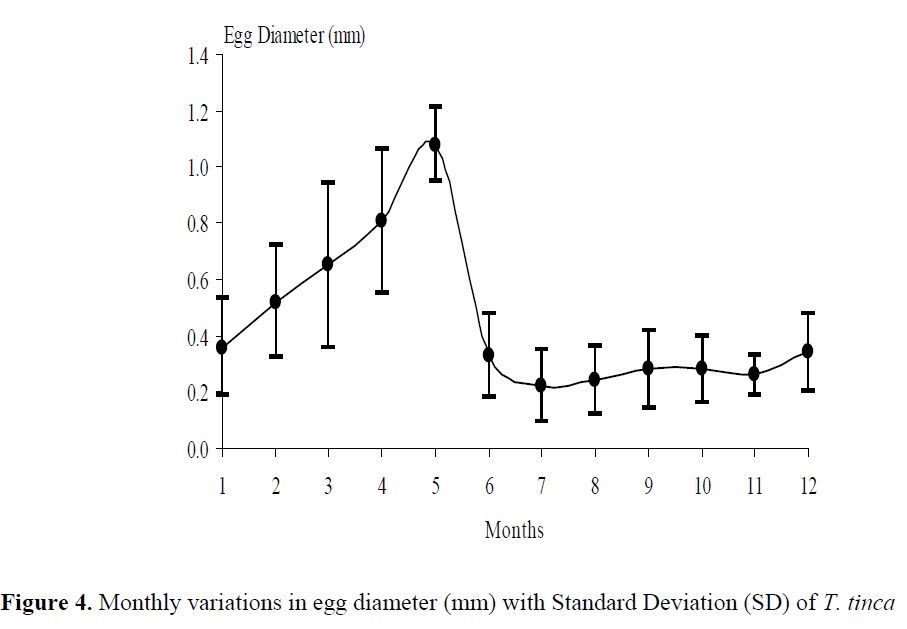
Figure 4. Monthly variations in egg diameter (mm) with Standard Deviation (SD) of T. tinca
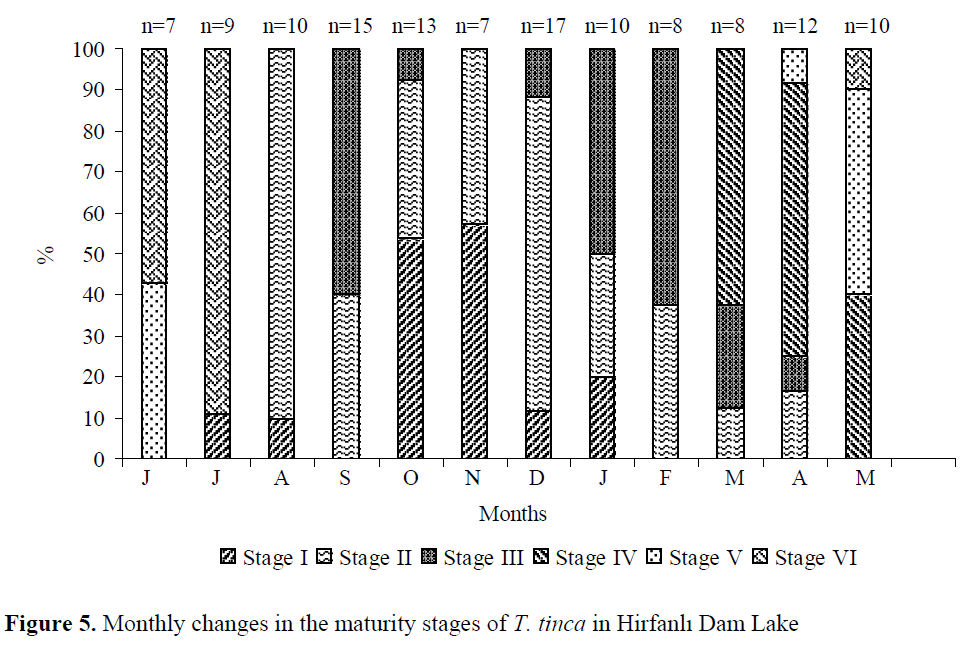
Figure 5. Monthly changes in the maturity stages of T. tinca in Hirfanl? Dam Lake
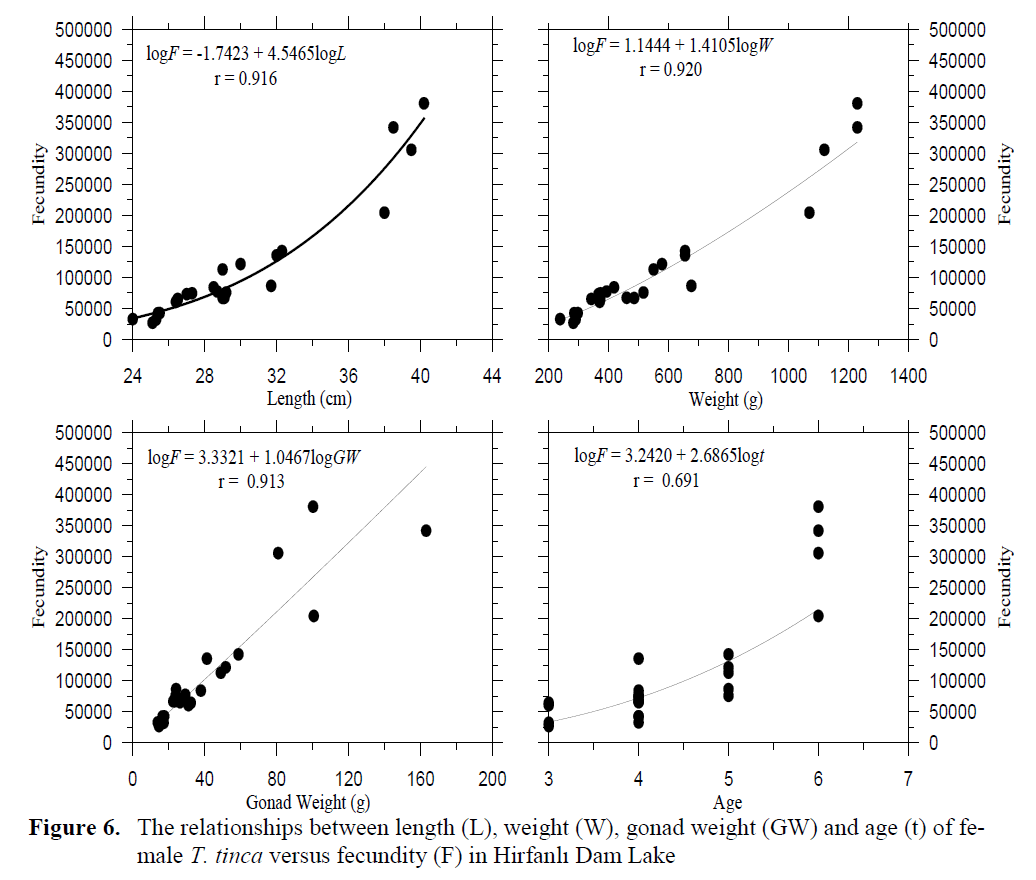
Figure 6. The relationships between length (L), weight (W), gonad weight (GW) and age (t) of fe-male T. tinca versus fecundity (F) in Hirfanl? Dam Lake
Fecundity
The number of oocytes of 24 females at ma-turity stages IV-V ranged from 27,010 (25.1 cm FL and 282 g body weight) to 380,639 (40.2 cm FL and 1230 g body weight), while mean abso-lute fecundity was 113,305 ± 97,826 (S.D). The value of relative fecundity was 347 ± 228 eggs mm-1 of fork length (minimum value was 108 and maximum 947 eggs mm-1) and 184 ± 52 eggs g-1 of total body weight (minimum value was 96 and maximum 309 eggs g-1) (Table 2). Fecundity was positively correlated with length, weight, gonad weight and age of fish. Older and larger fish showed a higher fecundity. The relationships between fecundity (F) – fork length (L), fecun-dity – weight (W), fecundity – gonad weight (GW) and fecundity – age (t) were described as follows:
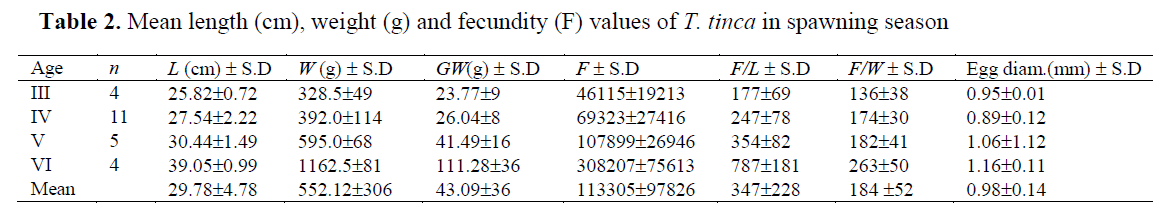
Table 2. Mean length (cm), weight (g) and fecundity (F) values of T. tinca in spawning season
log F= -1.7423 + 4.5465 logL (r= 0.916),
log F= 1.1444 + 1.4105 logW (r= 0.920),
log F= 3.3321 + 1.0467 logGW (r= 0.913),
log F= 3.2420 + 2.6865 logt (r= 0.691) (Fig-ure 6).
Mean egg diameter was 0.98 ±0.14 (SD) mm in the spawning season (Table 2). Oocytes were also observed in all seasons. The highest mean egg diameter was measured in May (1.08 mm) while the smallest diameter was measured in July (0.22 mm) during the study period (Figure 4).
The tench population of Hirfanl? Dam Lake shows a sex ratio of 0.92 males: 1.00 females, which is not significantly different from 1:1, nor much different from sex ratios reported in other studies (Y?lmaz, 2002; Ala? and Solak, 2004). In the present study, the ages of fish ranged from I to VI years and the dominant age group in the population was age III (33.1%). The abundance of age groups older than IV decreased dramati-cally and no fish were caught older than VI. This age bias may reflect the fishing customs practiced in and around the Hirfanl? Dam.
Spawning took place from the end of May (19 ºC) to the mid of July (25.8 ºC), according to monthly variations of GSI and egg diameter val-ues. In similar studies, Y?lmaz (2002) and Benzer et al. (2007) reported spawning period from late April to early July, in Porsuk Dam Lake and Hirfanl? Dam Lake, respectively. Other studies show slightly different periods and temperatures for spawning: O’maoileidigh and Bracken (1989) reported spawning from mid-June to the end of July in Ireland. Neophitou (1995) reported that spawning occurred during May and June at a water temperature of 18-20 ºC in Lake Pamvo-tida, Greece. Carral et al. (2003) and Ala? and Solak (2004) reported spawning between June and July (16.1 to 19.2 ºC). These comparisons reveal that tench populations, despite being geo-graphically widespread, often spawn at similar times, and are likely to be under the influence of local environmental factors. For example, fish transferred from their native habitat to a new one often show some variations in spawning charac-teristics, presumably as a result of slight changes in ecology (Nikolsky, 1963).
In the present study, age at sexual maturity of male and female fish was III and IV years, re-spectively, which is similar to the results of Y?lmaz (2002) and Ala? and Solak (2004). Neo-phitou (1995) and Benzer et al. (2007) reported that age of sexual maturity was III years for both sexes in Lake Pamvotida and Hirfanl? Dam Lake, respectively. In Ireland, tench in their third year, irrespective of sex, were still immature (O’maoileidigh and Bracken, 1989). Age of sex-ual maturity in tench may show variations in re-lation to water system, ecological characteristics and geographical location; spawning is often highly correlated with water temperature (O’maoileidigh and Bracken, 1989; Rodríguez et al., 2004).
The absolute fecundity of Lake Hirfanl? tench varied from 46115 ± 19213 to 308207 ± 75613 eggs/female, while mean relative fecundity was 347 ± 228 eggs mm-1 of fork length and 184 ± 52 eggs g-1 of total body weight. Absolute fecundity values ranged from 13,766 to 43,148 in Porsuk Dam Lake (Y?lmaz, 2002) and from 12,088 to 37,150 in Hirfanl? Dam Lake (Benzer et al., 2007). Absolute fecundity of tench in Hirfanl? Dam Lake was found higher than in Porsuk Dam Lake (Y?lmaz, 2002), in Hirfanl? Dam Lake (Benzer et al., 2007), in Kayabo?az? Dam Lake (Ala? and Solak, 2004), and in Ireland (O’maoileidigh and Bracken, 1989), being simi-lar to that in Lake Patryki, Poland (Pimpicka, 1981) and Lake Drweckie, Poland (Pimpicka, 1991). Relative fecundity reported by Y?lmaz (2002), varied from 57.60 to 132.36 mm?1 of av-erage length and from 63.73 to 100.24 g?1 of av-erage weight, and stated by Benzer et al. (2007), was in range of 47.40 - 116.82 mm?1 of average length and 40.29 - 56.03 g?1 of average weight. Relative fecundity value was found higher than in Porsuk Dam Lake (Y?lmaz, 2002) and in Hirfanl? Dam Lake (Benzer et al., 2007), being similar to that in Lake Patryki (Pimpicka, 1981) and Lake Drweckie (Pimpicka, 1991). Fecundity was sig-nificantly correlated to fish length, weight, gonad weight and age as reported in many studies (Pim-picka, 1981 and 1991; Y?lmaz, 2002; Türkmen et al., 2002; Ala? and Solak, 2004). Fecundity val-ues may be correlated with factors such as fish age, size, health, egg diameter, population den-sity, and a variety of environmental variables that affect the trophic status of a lake (Nikolsky, 1963). Any change in the environment (food supply, temperature, the amount of light, etc.) may result in significant changes in fecundity (Bagenal and Braum, 1978; Nikolsky, 1963).
Mean egg diameter in the spawning period was 0.98 ± 0.14 (SD) mm. Pinillos et al. (2003) reported that the diameter of mature oocytes was 0.7-0.8 mm in the spawning time, July. Mean egg diameter was reported as 0.9 mm in Lake Pam-votida (Neophitou, 1995). The egg radii were found to range between 0.4- 1.3 mm in Hirfanl? Dam Lake (Benzer et al., 2007). Egg diameter may be affected by fish size, gonad maturation stage and different parts of ovary as occurs in other species (Johnston and Leggett, 2002).
Fecundity-length, weight, gonad weight and age relationship parameters as logarithmic equa-tions are given in Table 3 to compare the results of present study to different water bodies. The relationships between absolute fecundity and the parameters under study showed that absolute fe-cundity significantly correlated to length, body weight and gonad weight and almost linear rela-tionship were found between them (see Table 3). These patterns were similar to the results of Pim-picka (1981 and 1991). In Ireland, regression coefficient showed that the relationship between total number of yolked eggs significantly corre-lated to total body weight (r= 0.91), fork length (r= 0.87) and total gonadal weight (r= 0.99) using a least squares regression analysis (O’maoileidigh and Bracken, 1989).
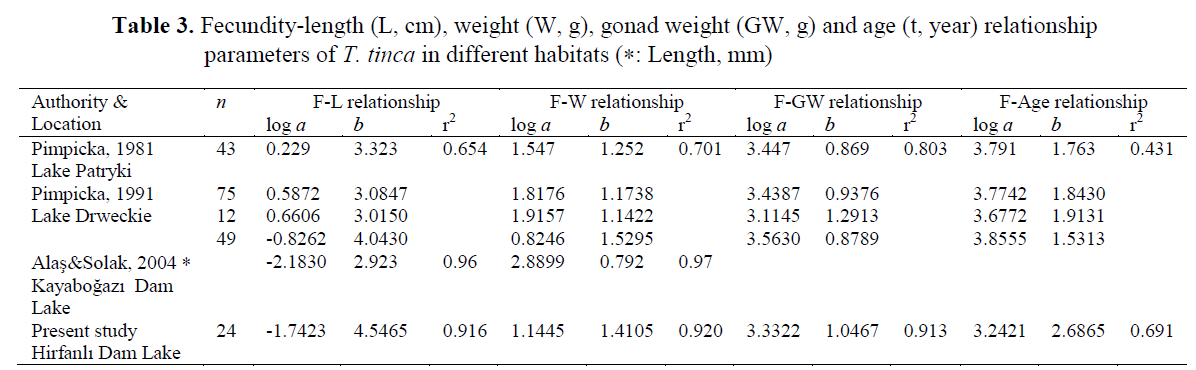
Table 3. Fecundity-length (L, cm), weight (W, g), gonad weight (GW, g) and age (t, year) relationship parameters of T. tinca in different habitats (∗: Length, mm)
Conclusions
In the present study, tench showed higher fe-cundity than that of the results of the other stu-dies carried out in Turkey. When the spawning time, first maturity age, fecundity, etc. were compared with the ones of Benzer et al. (2007) in Hirfanl? Dam Lake, the only spawning time showed similarity with this research. Curiously, over the course of the yearlong study, no tench
were captured older than VI age group. This might indicate a size bias in Hirfanl? Dam Lake due to overfishing. However, since tench reach reproductive maturity at ages III-IV, this would not appear to negatively affect the population characteristics of the lake, meaning tench are not poached prior sexual maturity and off-season harvesting.
Acknowledgements
I thank Dr. Rick Hochberg at the University of Massachusetts for help with the comments on this manuscript.
765
References
- Adámek, Z., Sukop, I., Moreno Rendón, P., Kou-ril, J., (2003). Food competition between 2+ tench (Tincatinca L.), common carp (Cypri-nus carpio L.) and bigmouth buffalo (Icti-obuscyprinellus Val.) in pond polyculture, Journal of Applied Ichthyology, 19: 165-169. doi:10.1046/j.1439-0426.2003.00467.x
- nAlaş, A., Solak, K., (2004). The reproductive bi-ology of the tench (Tincatinca L., 1758) in Kayaboğazı Dam Lake, Turkish Journal of Veterinary and Animal Sciences, 28: 879-885
- nAltındağ, A., Shah, S. L., Yiğit, S., (2002). The growth features of tench (Tincatinca L., 1758) in Bayındır Dam Lake, Ankara, Tur-key, Turkish Journal of Zoology, 26: 385-391.
- nBagenal, T. B., Braum, E., (1978). Eggs and early life history. In: Bagenal, T. B. (Ed.), Methods for assessment of fish production in freshwaters. 3rd ed. IBP Handbook Blackwell, Oxford, pp. 165-201.
- nBalık, I., Çubuk, H., (2000). Efficiency of cap-ture of tench, Tincatinca L. by trammel nets of monofilament net twine combinations, Fisheries Management Ecology, 7: 515-521. doi:10.1046/j.1365-2400.2000.00216.x
- nBalık, S., Sarı, H. M., Ustaoğlu, M. R., İlhan, A., (2004). The structure, mortality and growth of the tench (Tincatinca L., 1758) popula-tion in Çivril Lake (Denizli, Turkey), Tur-kish Journal of Veterinary and Animal Sciences, 28: 973-979 (in Turkish).
- nBeklioğlu, M., Moss, B., (1998). The effects of tench (Tincatinca (L.)) and sticklebacks (Gasterosteusaculeatus L.) on planktonic and benthic communities in mesocosms in a shallow lake, Aquatic Ecology, 32: 229-240. doi:10.1023/A:1009946505397
- nBenzer, S. Ş., Gül, A., Yılmaz, M., (2007). Breeding properties of Tincatinca (L., 1758) Living in Hirfanlı Dam Lake (Kırşehir, Turkey), Ege University Journalof Fisheries &Aquatic Sciences, 24 (1-2): 127-129
- nCarral, J. M., Rodríguez, R., Celada, J. D., Sáez-Royuela, M., Aguilera, A., Melendre, P., (2003). Successful gonadal development and maturation of tench (Tincatinca L.) in small concrete ponds, Journal of Applied Ichthy-ology, 19: 130-131. doi:10.1046/j.1439-0426.2003.00458.x
- nDSI, (1973). Limnological report on Hirfanlı Dam Lake (HirfanlıBarajGölü’nün Lim-nolojikraporu). Devlet Su İşleriGenelMüdürlüğü, Ankara, 51 pp (in Turkish)
- nErgönül, M. B., Altındağ, A., (2005). The occur-rence and dynamics of Ligula intestinalis in its cyprinid fish host, Tincatinca, in Mogan Lake (Ankara, Turkey), VeterinarniMedi-cina, 50: 537-542
- nHerrero, M. J., Pascual, M., Madrid, J. A., San-chez-Vazquez, F. J., (2005). Demand feed-ing rhythms and feeding-entrainment of lo-comotor activity rhythms in tench (Tincatinca), Physiology & Behavior, 84: 595-605. doi:10.1016/j.physbeh.2005.02.015
- nJohnston, T. A., Leggett, W. C., (2002). Maternal and environmental gradients in the egg size of an iteroparous fish, Ecology, 83: 1777-1791.doi:10.1890/0012-9658(2002)083[1777:MAEGIT]2.0.CO;2
- nLagler, K. F., (1956). Freswater Fishery Biology. W. M. C. Brown Company, Dubuque, Iowa,421 pp
- nNeophitou, C., (1995). Some biological data on tench (Tincatinca (L.)) in Lake Pamvotida, Greece, ActaHydrobiologica, 35: 367-379.
- nNikolsky, G. V., (1963). The Ecology of Fishes. Academic Press, London, 352 pp. O’maoileidigh, N. and Bracken, J. J. (1989). Bi-ology of the tench, Tincatinca (L.), in an Irish lake, Aquaculture and Fisheries Man-agement, 20: 199-209
- nPimpicka, E., (1981). Fecundity of tench (Tincatinca L.) in Lake Patryki, ActaIchthyologicaetPiscatoria, 11: 3-19. Pimpicka, E., (1991). Fecundity of tench (Tincatinca L.) females in Lake Drweckie, ActaIchthyologicaetPiscatoria, 21: 129-141.
- nPinillos, M. L., Delgado M. J., Scott A. P., (2003). Seasonal changes in plasma gonadal steroid concentrations and gonadal mor-phology of male and female tench (Tincatinca L.), Aquaculture Research, 34(13): 1181-1189. doi:10.1046/j.1365-2109.2003.00926.x
- nRodríguez, R., Celada, J. D, Sáez-Royuela, M., Carral, J. M., Aguilera, A., Melendre, P. M., (2004). Artificial reproduction in 1-year-old tench (Tincatinca L.), Journal of Applied Ichthyology, 20: 542-544. doi:10.1111/j.1439-0426.2004.00584.x
- nSaila, S. B., Recksiek, C. W., Prager, M. H., (1988). Fishery Science Application System, A Compendium of Microcomputer Pro-grams and Manual of Operation. Elsevier, New York, 223 pp.
- nSümbüloğlu, K., Sümbüloğlu, V., (1994). Bios-tatistics (Biyoistatistik), ÖzdemirYayıncılık, Ankara, 269 pp (in Turkish).
- nSvobodova, Z., Kolarova, J., (2004). A review of the diseases and contaminant related mortal-ities of tench (Tincatinca L.), VeterinarniMedicina, 49: 19-34
- nTürkmen, M., Erdoğan, O., Yıldırım, A., Akyurt, I., (2002). Reproduction tactics, age and growth of CapoetacapoetaumblaHeckel 1843 from the Aşkale region of the Karasu River, Turkey, Fisheries Research, 54: 317-328. doi:10.1016/S0165-7836(01)00266-1
- nWedekind, H., Rennert, B., Kohlmann, K., (2003). Product quality in different strains of tench (Tincatinca) tested under controlled environmental conditions, Journal of Ap-plied Ichthyology, 19: 161-164. doi:10.1046/j.1439-0426.2003.00457.x
- nYılmaz, F., (2002). Reproductive biology of the tenchTincatinca (L., 1758) inhabiting Por-suk Dam Lake (Kütahya, Turkey), Fisheries Research, 55: 313-317. doi:10.1016/S0165-7836(01)00294-6















