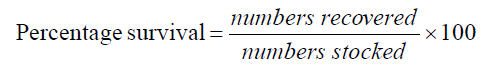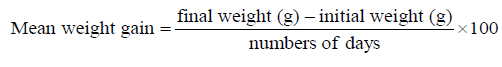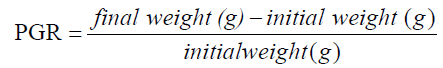Keywords
Captive; Rearing; Ornamental fish; Arunachal Pradesh; India
Introduction
Ornamental fish, also known as aquarium fish and they are the live jewels and the most attractive living organisms of the aquatic world. Their lively and fascinating activities are worth enjoying as colourful fish has high aesthetic value. Throughout the world, ornamental fish keeping is very popular as interior decorative materials; an easy and stress relieving hobby. Besides home aquaria, public aquarium in hotels, parks and other public places are common in metropolis. About 7.2 million houses in the USA and 3.2 million in the European Union have an aquarium and the number is increasing day by day throughout the world (Ghosh et al., 2003). As of now, the demand for fish has been increasing due to not only the increase in population but also aquarium fish trade and their values towards health benefits of human being. As fish farming intensifies and artificial propagation of the ornamental fish, so rearing feasibility is top priority as well as providing feed that are nutritionally balanced for the utmost growth in the aquarium.
Now a day, ornamental fish rearing in aquarium is so popular in urban India, in comparison to rural areas due to lack of awareness of such culture. Northeastern India has many species of fish that have great potential in the ornamental trade and many of them are attractive to foreign markets. There is great potential to expand this industry. At present production level of aquarium fishes does not cope with the demand of domestic market. Moreover, culture of the indigenous ornamental fish species can generate a source of income to the economically weaker section and the educated unemployed youth. There is hardly reported on the captive rearing of ornamental fishes in the region. Notably, (Dakua et al., 2013) investigated on the rearing feasibility of Esomus danricus and Parluciosoma daniconius from upper Assam, India. Therefore, the present study has been taken up on the captive rearing of certain wild ornamental fishes.
Materials and Methods
The experiment was conducted at the Fish and Aquatic Ecology laboratory of Department of Zoology, Rajiv Gandhi University, Rono Hills during October 2015 to May 2016. A total of different 11 (eleven) hill stream ornamental fish species were selected for this experiment. Live wild fishes were collected from sampling site of the Dikrong River. The coordinate of the site is N 27009′29.12″ and E 93045′26.45″ with 247 msl of Arunachal Pradesh (Figure 1). After collection, the specimen was treated with one spoon of NaCl or 2 to 3 drops of KMnO4 solution mixed in 16 liters of waters and kept for 2 days. Then transferred for standardize captive rearing in the cemented tank (Figure 2). Later, transferred all of them in their respective aquarium size of 120 × 60 × 50 cm3 and 60 × 40 × 45 cm3 maintain with a water depth of 25 cm for acclimatization having floating 2-3 weeds (Figure 3). Before transferred, the length and weight of the individual of each species by using divider and measuring board having graduation in mm and electronic balance was nearest to 0.01 gm. The number of each species varies from one aquarium to other and for which 11 numbers of aquaria were used. Fishes were provided with artificial food such as freeze dried tubifex, grinded dry small fish (powder) and small fine floating pellets for 2 or 3 times daily and sometimes mosquito larvae. Growth rate in terms of length and weight were monitored by maintaining standard water quality and providing artificial fish feed periodically. The water quality of aquarium was constantly monitored on monthly basis as per procedure of APHA (1992). The survival and mean growth rate of the fishes were calculated by the following formula (Francis, 1995). Similarly, percentage growth rate (PGR) was determined by the following formula of (Sakthivel and Bhaskaran, 1995):
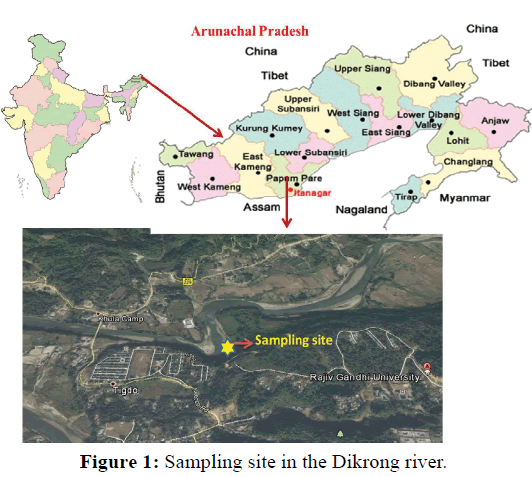
Figure 1: Sampling site in the Dikrong river.

Figure 2: Cemented tank (for temporary acclimatization).

Figure 3: Aquarium set for rearing of fish species.
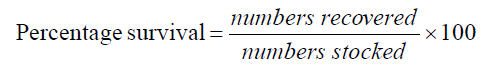
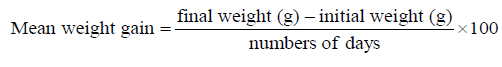
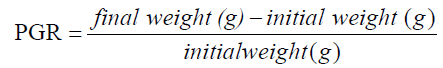
Results and Discussion
Rearing feasibility of selected species
The rearing practice for selected fish species was shown in Table 1. For this experiment, different aquarium was used for each species. Devario aequipinnatus, while introduced, it was measured 72.5 mm (± 0.02) in average body length with 4.14 g (± 0.04) body weight and reached up to 75.6 mm (± 0.02) in length with 4.71 g (± 0.03) weight. Garra sp., the average initial length was 53.07 (± 0.01) mm and grows up to 53.1 (± 0.01) mm and their average weight 1.62 g (± 0.01) increases to 1.6 g (± 0.02). Pethia ticto, the average initial length was 93.1 mm (± 0.01) and grows up to 93.6 mm (± 0.01) and their average initial weight was 11.0 g (± 0.01) and increases to 11.1 g (± 0.01). Mystus dibrugarensis, the average initial weight was 4.42 g (± 0.02) with average length 81.2 mm (± 0.02) and attain a growth up to 4.57 g (± 0.03) in weight and 83.7 mm (± 0.02) in length. Lepidocephalichthys guntea, the initial average length was 60.9 mm (± 0.01) with the average weight was 1.45 g (± 0.01) and finally measured its length 61.0 mm (± 0.01) and weight 1.5 g (± 0.01). Barilius bendelisis, it was measured 106.6 mm (± 0.01) as initial average body length with 11.55 g (± 0.01) body weight and reached up to 107.7 mm (± 0.01) in length with 11.6 g (± 0.01) in weight. Aborichthys kempi, the average initial length was 84.7 mm (± 0.01) and grows up to 85.9 mm (± 0.01) while their average initial weight was 2.75 g (± 0.01) and increases to 2.85 g (± 0.01). Botia rostrata, the average initial weight was 2.55 g (± 0.01) with average length 69.1 mm (± 0.01) and attains a growth up to 69.2 mm (± 0.01) in length with 2.67 g (± 0.01) in weight. Amblyceps apangi, the initial average length was 84.2 mm (± 0.03) with the average weight 3.84 g (± 0.02) and finally measured its length as 85.8 mm (± 0.04) and weight as 4.0 g (± 0.03). Danio rerio, the initial average length was 39.3 mm (± 0.00) and grows up to 39.3 mm (± 0.00) while their average weight was 0.5 g (± 0.00) and remains up to 0.5 g (± 0.00). Olyra praestigiosa, the average initial length was 81.3 mm (± 0.03) and remains grow up to 81.3 mm (± 0.03) and their average initial weight was 1.44 g (± 0.02) and increases to 1.30 g (± 0.03).
| Name and No. of specimens |
Average Weight (g) |
Average length (mm) |
Mean weight gain (%) |
% survival |
PGR |
Feed/fish (g) |
| Initial |
Final |
Student’s t Test |
Initial |
Final |
Student’s t Test |
| D. aequipinatus (N=15) |
4.14 ±0.04 |
4.71 ±0.03 |
-3.426 |
72.5 ±0.02 |
75.6 ±0.02 |
-7.535* |
1.9 |
100 |
0.137 |
0.13 |
| Garrasp. (N=4) |
1.62 ±0.01 |
1.69 ±0.02 |
-6.545* |
53.07 ±0.01 |
53.1 ±0.01 |
-4.276* |
1.633 |
100 |
0.043 |
0.048 |
| P. ticto (N=4) |
11.0 ±0.01 |
11.1 ±0.01 |
-2.596 |
93.1 ±0.01 |
93.6 ±0.01 |
-3.449* |
0.33 |
100 |
0.009 |
0.33 |
| M. dibrugarensis (N=10) |
4.42 ±0.02 |
4.57 ±0.03 |
-8.211* |
81.2 ±0.02 |
83.7 ±0.02 |
-13.408* |
0.5 |
100 |
0.034 |
0.132 |
| L. guntea (N=2) |
1.45 ±0.01 |
1.5 ±0.01 |
-19* |
60.9 ±0.01 |
61.0 ±0.01 |
-1.0 |
0.17 |
100 |
0.034 |
0.04 |
| B. bendelisis (N=4) |
11.55 ±0.01 |
11.6 ±0.01 |
-3.068 |
106.6 ±0.01 |
107.7 ±0.01 |
-4.392* |
0.33 |
100 |
0.004 |
0.342 |
| A. kempi (N=3) |
2.75 ±0.01 |
2.85 ±0.01 |
-3.464 |
84.7 ±0.01 |
85.9 ±0.01 |
-2.412 |
0.33 |
100 |
0.036 |
0.08 |
| B. rostrata (N=2) |
2.55 ±0.01 |
2.67 ±0.01 |
-1.769 |
69.1 ±0.01 |
69.2 ±0.01 |
-10.0 |
0.4 |
100 |
0.047 |
0.076 |
| A. apangi (N=13) |
3.84 ±0.02 |
4.0 ±0.03 |
-7.053* |
84.2 ±0.03 |
85.8 ±0.04 |
-10.292* |
0.53 |
100 |
0.042 |
0.104 |
| D. rerio (N=2) |
0.5 ±0.00 |
0.5 ±0.00 |
-- |
39.3 ±0.00 |
39.3 ±0.00 |
-- |
0 |
100 |
0 |
0.015 |
| O. praestigiosa(N=16) |
1.44 ±0.02 |
1.30 ±0.03 |
-3.659* |
81.3 ±0.03 |
81.3 ±0.03 |
0.009 |
-0.47 |
37.5 |
-0.097 |
0.04 |
| * Significant at the 0.05 level; without * not Significant. |
Table 1: Growth performance, food intake and survivability rate of 11 wild ornamental fishes.
The Student’s test (t) of significant for the differences between initial and final weights and lengths of all the species has calculated (Table 1). At the 0.05 level, the values for initial and final weight of the Garra sp. was found to be significant (t=-6.545); the initial and final weight of M. dibrugarensis was also significant (t=-8.211); the initial and final weight of L. guntea was significant (t=-19); the initial and final weight of A. apangi was also significant (t=-7.053) and the initial and final weight of O. praestigiosa was significant (t=-3.659). The differences between initial and final weights of the other fish species were not significant at 0.05 level. In case of the length, the values of initial and final of D. aequipinatus was found to be significant (t=-7.535); the values of initial and final of Garra sp. was significant (t=-4.276); the values of initial and final of P. ticto was significant (t=-3.449); the values of initial and final of M. dibrugarensis was significant (t=-13.408); the values of initial and final of B. bendelisis was significant (t=-4.392) and the values of initial and final of A. apangi was also significant (t= -10.292). The differences between initial and final length of the other species were not significant at 0.05 level.
The mean weight gain was found to be highest (1.9%) in D. aequipinnatus and followed by Garra sp. (1.633%) while, the lowest mean weight gain was observed in O. praestigiosa (-0.47%) and D. rerio (0.0%). The other species like P. ticto, M. dibrugarensis, L. guntea, B. bendelisis, A. apangi, B. rostrata and A. kempi were also recorded their slight weight gain. In case of percentage growth rate (PGR), the maximum (0.137) was recorded in D. aequipinatus followed by Garra sp. (0.043) and its minimum was in O. praestigiosa (-0.097) and D. rerio (0.0). The average PGR were observed in the remaining species viz. P. ticto, M. dibrugarensis, L. guntea, B. bendelisis, A. apangi, B. rostrata and A. kempi. Based on these aspects, O. praestigiosa was not successfully reared in spite of best effort while, D. rerio was found neither weight gain nor increase of percentage growth rate during the rearing practice. Hence, O. praestigiosa and D. rerio was not fit in the aquarium condition from the ornamental fish point of view.
Survival rate and timing of feed provided
In overall, the survival rate for all species was found to be 100% except O. praestigiosa and indicating that they can easily acclimatized and survived in aquarium condition for long time. Similar results were also observed by Singh (2011) and Dakua et al. (2013) for various ornamental fishes from the Brahmaputra River. In case of O. praestigiosa, the survivality rate was 37.5% as out of the 16 specimens only 6 of them has been survived. The feed supplement was provided at 3% body-weight of individual species mainly during morning and evening (daily 2-3 times). As the fish increase in size the proportion of feed required for maintenance of species also increased (Pillai and Lakra, 2000). In overall of rearing, the provided supplementary feed rate was ranged from 0.015 to 0.342 mg. The lowest feed rate was recorded in D. rerio while its highest was in B. bendelisis. Supplied artificial diets are tokyo feed (Discus Thailand), tubifex (Royal Feast) and other natural live feed (earthworm and mosquito larva). Most of the reared fishes preferred tubifex in comparison to other feeds. The result reveals that they can grow well and easily acclimatize in aquarium condition except O. praestigiosa.
Further, it is clear that, out of the 11 experimental species, O. praestigiosa was found to be unfit for rearing in the aquarium condition due to their poor response in respect of the length and weight. It was repeatedly tried to attempt the domestication of the O. praestigiosa in the aquarium condition but its outcome was negative as it difficult to maintain their natural habitat. However, the rest of the species were found to be suitable for rearing and could be considered as hill stream ornamental fish species.
Practically, attempt to domestication on certain species could also help in the artificial propagation of endangered species.
Physico-chemical parameters of the tank and aquarium
The water quality of the tank and aquarium has been monitored every month from October to May (Table 2). The 50% of the water volume in aquarium was changed after every month. The water temperature ranged from 14°C to 27°C, as the lowest was observed in December (stocking tank) and highest in May (medium aquarium). pH varied between 6.8 (Apr) and 7.6 (Jan) and the lowest was recorded in stocking tank and highest in large aquarium. Dissolved oxygen (DO) found within 6.55 to 7.91 ppm and the highest was observed in cemented tank and the lowest in medium aquarium. Free carbon dioxide (CO2) concentration (1.0 ppm) was almost same trend in tank and aquaria while, total alkalinity ranged from 65 to 76 and the maximum was observed in large aquarium and the minimum in cemented tank during April and December respectively. The finding values of the water quality were found within the permissible limit. A pH range of 6.5 to 9.0 is suitable for fish culture (Hora and Pillay, 1962); dissolved oxygen within 5 to 8 ppm, as the concentration was above 5 ppm is favourable for most fish species (Lichtenberger, 1985). Dakua et al. (2013) was also observed the similar results in the rearing of ornamental fish in the aquarium.
| Parameters |
Stocking tank |
Aquarium 1 (Large-size) |
Aquarium 2 (Medium-size) |
| Water Temperature (°C) |
14 -24 |
14-27 |
14-27 |
| pH |
6.8-7.5 |
7.0-7.6 |
7.1-7.5 |
| Dissolved oxygen (ppm) |
6.98-7.91 |
6.88-7.69 |
6.55-7.12 |
| CO2 (ppm) |
1.0 |
1.0 |
1.0 |
| Alkalinity (ppm) |
65-72 |
70-76 |
70-72 |
Table 2: Parameters of the aquarium for captive rearing experiments.
Conclusion
From the above findings, it reveals that as the length of fish increase with slight increases of weight in each species except O. praestigiosa and D. rerio. In overall, the survival rate was found to be cent percent (100%) for most of the species. The water quality of the tank and aquarium was monitored constantly during the experiment because survivability of the fish also depends on the water condition. It indicates that they can easily acclimatize and survive in aquarium condition for long time. The prevailing environmental factors are suitable for culture and propagation of the rearing species in aquarium condition. Ornamental fish farming/rearing can be a promising alternative for local people of the region from trading point of view.
Acknowledgements
The authors are thankful to Department of Biotechnology, New Delhi, Govt. of India for financial help through DBT Project No. BT/382/NE/TBP/2012 to carry out the work at the Live Fish Germplasm Bank of Department of Zoology, Rajiv Gandhi University, Rono Hill, Itanagar. The third author (AD) is also thankful to the Centre with Potential for Excellence in Biodiversity, Rajiv Gandhi University, Doimukh for fellowship.
17964
References
- APHA (1992) Standard methods for the examination of water and wastewater. 18th Edition,APHA Washington, USA.
- Dakua, S., Abujam, S.K.S., Paswan, G., Bordoloi, R. (2013) Rearing feasibility of Esomus danricus (ham.) and Parluciosoma daniconius (Ham.) in aquarium. J Indian Fisheries Association40, 89-92.
- Francis, O.N. (1995) Hatchery Propagation of Five Hybrid Groups by Artificial Hybridization of Clarias gariepinus (B.) and Heterobranchus longifillis (Val.) Clariidae, using Dry Powdered Carp Pituitary Hormone. JAquacultTrop10, 1-11.
- Ghosh, A., Mahapatra, B.K., Datta, N.C. (2003) Ornamental fish farming-successful small scale aqua business in India. AquaAsia 3, 14-16.
- Hora, S.L.,Pillay, T.V.R. (1962) Handbook on Fish Culture in the Indo-Pacific Region. FAO Fisheries Technical Paper 14,p: 204.
- Lichtenberger,M. (1985) Husbandary of the Barred spiny eel, Macrognathus pancalus. Journal of Tropical Medicine and Hygiene 88, 169-174.
- Pillai, A.B., Lakra, W.S. (2000) Intensive rearing of mahseer (Tor khudree) fry under controlled hatchery conditions. Singh, H.R. and LakraW.S. (Eds.). Coldwater Aqua and Fisheries,229-234.
- Sakthivel, M., Bhaskaran, P.(1995) Effects of Dietary Carbohydrate on Growth, Feed Conversion, Protein Utilization and Body Carcass of a Common Air-breathing Freshwater Teleost Fish Channa punctatus (Bloch). JAquacultTrop10,119-127.
- Singh, S.K.A. (2011) Studies on ecology, biology and rearing feasibility of two Spiny Eel, Macrognathus aral (Bloch and Schneider) and Macrognathus pancalus (Hamilton-Buchanan) from Upper Assam. Unpublished, Ph.D Thesis, Dibrugarh University.
- Tiamiyu, L.O., Okomoda V.T., Agbese, V.E. (2015) Growth performance of Clarias gariepinus fingerlings fed Citrullus lanatus seed meal as a replacement for soybean meal. J of Aquaculture Engineering and Fisheries Res1,49-56.n