Keywords
Cephalopod, Iskenderun Bay, Eastern Mediterranean, Biology, Distribution
Introduction
Cephalopods are found in all the oceans of the world and at all depths. There are numerous studies on cephalopods from the three major orders of Coleoidea (Octopoda, Sepioidea, Teuthoidea), that live on the continental shelf (Boyle and Daly, 2000). These species are abundant and ecologically important and a deep knowledge in their distribution and biology is undoubtedly important. Cephalopod fisheries are largely unregulated and the exploited populations show marked inter-annual fluctuations unrelated to fishery landings and effort in European waters. But it is also necessary that enough data from new sampled areas in the Mediterranean Sea will be available for an accurate identification of species distribution.
Geographic and bathymetric distributions of cephalopods have been studied in detail in different areas; recent studies in different parts of the Mediterranean Sea, (Kallianiotis et al., 2000; Nishiguchi, 2000; Norman, 2000; Quetglas et al, 2000; Lefkaditou et al., 2001; Machias et al, 2001; Gonzalez and Sanchez, 2002; Lloret and Lleonart, 2002; Tserpes and Peristeraki, 2002; Rawag et al, 2004; Gaertner et al., 2005; Massuti and Renones, 2005) and the Turkish coasts of Mediterranean Sea (Salman et al., 2000; Salman et al., 2002; Salman et al., 2003; Salman and Katagan, 2004).
Iskenderun Bay is located on the North-East end of the Eastern Mediterranean with an area of approximately 2 275 km2, a length of 65 km and a width of approximately 35 km. The eastern Mediterranean Sea was known to be a rich fishery relative to the Mediterranean standard. The local continental shelf in Iskenderun Bay, in which nearly all fishing activity occurs, is relatively wide and its margins are bordered by relatively shallow water (10-40 m) as compared to other parts of the eastern Mediterranean Sea (Anonymous, 2002). The bay has an average depth of 70 m (Iyiduvar, 1986). Therefore, topographically, it is suitable for trawling (Anonymous, 2002).
Nowadays, cephalopod fisheries are increasing in the Mediterranean sea of Turkey (Anonymous, 2004). However, with the increase of fishing vessels, fish stocks are becoming over exploited. Pollution in the bay, due to the industrialization of the area, has also been a major reason for the decline of marine stocks (Anonymous, 2002). This work has been done in a new, non-studied area of the Mediterranean, and therefore represents an important in the knowledge of the distribution and biology of cephalopod fauna in the Mediterranean. This study was carried out to establish the species present in the Iskenderun Bay.
Materials and Methods
45 bottom trawls were carried out in March 2004 to May 2005, at depths from 30 to 200 m in Iskenderun bay (Eastern Mediterranean). At each month, 3 trawl operations were organized and sub sampling was carried out. All hauls were done during the normal fishing period during day-light. To reflect the fishing efforts of the Iskenderun bay, Karatas port has been chosen (Fig. 1). The samples were obtained from the Karatas port’s local fishing fleet, equipped with a typical Mediterranean type deep trawl (22 mm mesh size).
Figure 1: Studying area in Iskenderun Bay.
The samples were first kept in boxes which were full of ice just after capture and then transferred to the laboratory to store at -18 °C until identification and measurements. After the frozen samples were thawed at 4 °C overnight, they were identified according to procedures as described by Nesis, 1987; Roper et al., 1984 and Mangold & Boletzky, 1987. Than dorsal mantle length (DML), (to the nearest cm below), total weight (TW), (to the nearest g below) and determination of sex were carried out on each specimen.
Results and Discussion
During the sampling period a total of 1101 cephalopod individuals were captured belonging to 8 different species included in 3 different orders (Table 1). The species are listed in tables (1, 2), with the number of specimens captured in each month of sampling and biological parameters of cephalopod species. A total of 645 Sepia officinalis individuals caught (58.6% of total cephalopod catch) and they were more abundant in September, December, March and the last caught at May 2005. S. officinalis individuals were heaviest and longest in January 2005 and the smallest been caught in May 2005. Larger females and males appeared in October, and January. Eledone moschata specimens were caught at the most abundant (13.6%) of total catch. Larger males and females caught in April 2005. Larger females and males individuals of Loligo vulgaris sampled the same time with S. officinalis individuals and that specimen was the third most abundant species during the studying period. Illex coindetii individuals were the fourth most abundant of total catch (9%). And that specie was only sampled in a few months. The largest individuals were caught in early summer.
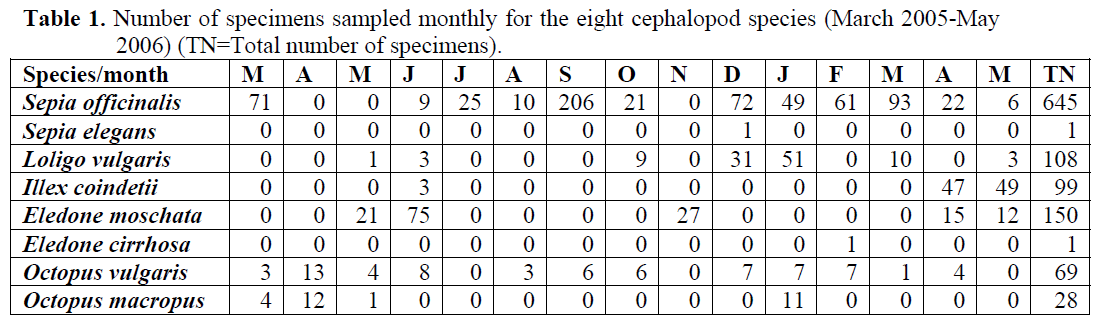
Table 1: Number of specimens sampled monthly for the eight cephalopod species (March 2005-May 2006) (TN=Total number of specimens).
Biology of some commercially important cephalopods
Sepia officinalis Linnaeus, 1758: A total of 645 specimens were captured. S. officinalis represented 58.6 % of the total sample. The minimum ML was 3.7 cm and the maximum ML was 20 cm. The mean ML±SE of the species was 10.74 ±0.10 cm (Table 2). A comparison between the growth slopes for males and females showed that growth parameters were statistically not significantly different, (Anova, p<0.05). Weights varied from 5.28 to 442.26 g, with a mean body weight (TW±SE) 126.42 ±3.24 g. During the study, larger specimens were more frequent in the end of the winter and early spring. Dorsal mantle length and total weight relationships of S. officinalis individuals were determined considering all the individuals, only males and only females (Table 3).
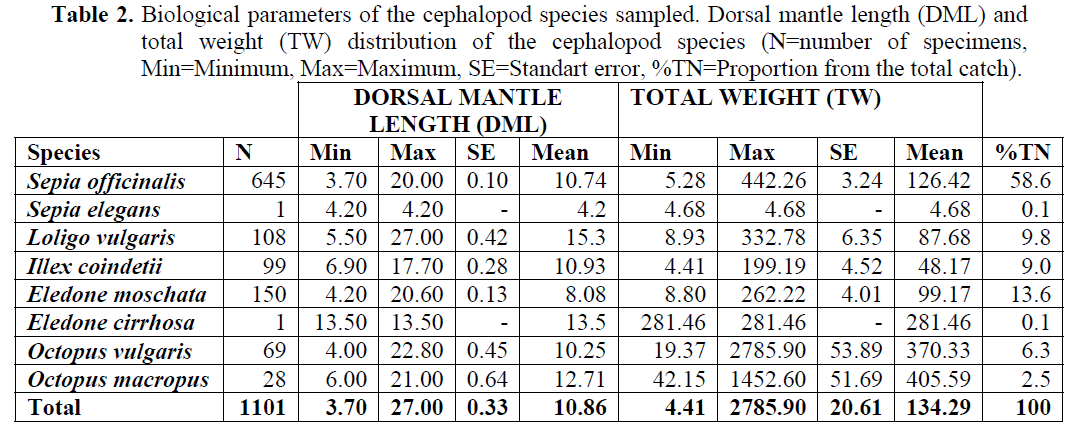
Table 2: Biological parameters of the cephalopod species sampled. Dorsal mantle length (DML) and total weight (TW) distribution of the cephalopod species (N=number of specimens, Min=Minimum, Max=Maximum, SE=Standart error, %TN=Proportion from the total catch).
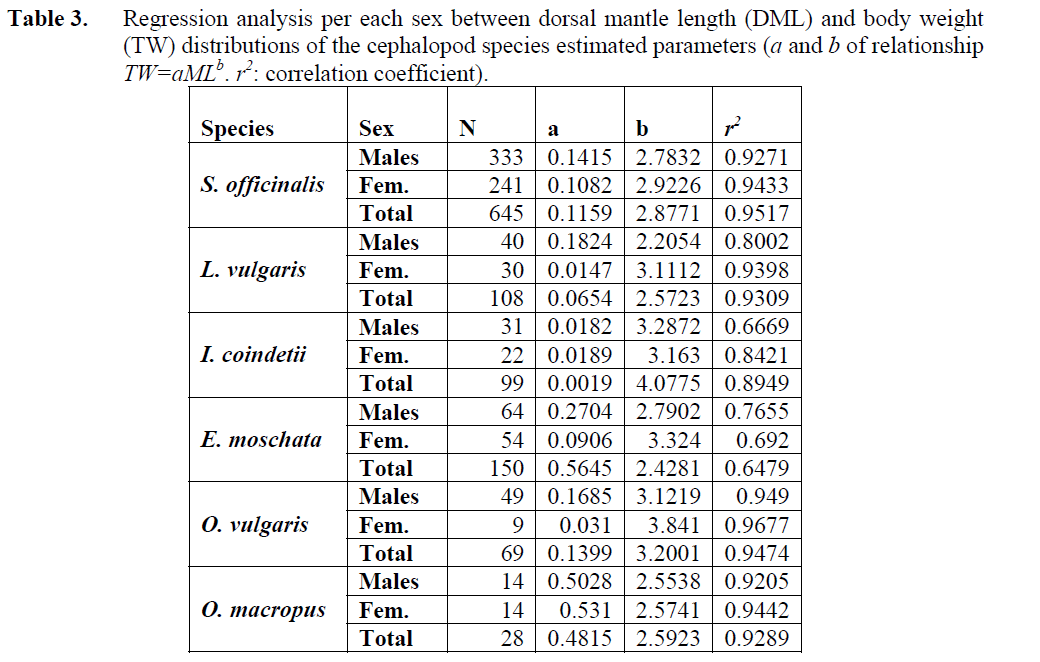
Table 3: Regression analysis per each sex between dorsal mantle length (DML) and body weight (TW) distributions of the cephalopod species estimated parameters (a and b of relationship TW=aMLb. r2: correlation coefficient).
L. vulgaris Lamarck, 1798: During the sampling period a total of 108 specimens of L. vulgaris were collected (May, June, October, December 2004, January and March 2005). Of these individuals, 40 were males with dorsal mantle lengths between 11.2 and 27 cm, 30 were females with ML between 10 and 23 cm. The mean dorsal mantle length (ML±SE) of the species was 10.93 ±0.42 cm. In this case, the comparison of growth slopes between males and females individuals did not provide evidence for significant differences between sexes (Anova, p<0.05). Mean value of total weight (TW±SE) for males and females also calculated as 87.68 ±6.35 g (Table 2). Dorsal mantle length and total weight relationships of L. vulgaris individuals were estimated for males, females and total (Table 3). During the study, larger specimens were more frequent in the end of the spring and early summer.
I. coindetii (Verany, 1839): A total of 99 Illex coindetii were caught. Of these were 31 males with dorsal mantle lengths of between 9 and 14.3 cm, and body weight between 16.59 and 135.45 g and 22 were females with ML of between 11 and 17.7 cm, with body weight between 25.99 and 199.19 g. The mean ML±SE of the species was 10.93 ±0.28 cm and TW±SE of the species 48.17 ±4.52 calculated (Table 2). Larger specimens were more frequent in the end of the winter and early spring. Dorsal mantle length and total weight relationships of I. coindetii individuals were estimated for males, females and total (Table 3).
Eledone moschata (Lamarck, 1799): A total of 150 individuals were captured. The maximum and minimum values of ML were 20.6 and 4.2 cm respectively. The mean dorsal mantle length (DML±SE) and the mean body weight TW±SE of the species were calculated as 8.08±0.13 cm and 99.17 ±4.01 (Table 2). During the study period, larger specimens were more frequent in the end of the winter and early spring. Dorsal mantle length and total weight relationships of E. moschata individuals were estimated for males, females and total (Table 3).
Octopus vulgaris Cuvier, 1797: A total of 69 specimens (49 males, 9 females and 11 unspecified) were captured. The ML length was 4 cm, maximum ML was 22.8 cm. The minimum body weight was 19.37 g and maximum 2785.90 g. The mean ML±SE and TW±SE of the specimens were calculated as 10.25 ±0.45 cm and 370.33 ±53.89 g (Table 2). During the study, larger specimens were more frequent in the end of the winter.
Octopus macropus Risso, 1826: A total of 28 specimens were captured during the study period. The minimum and maximum values of dorsal mantle length were 6 and 21 cm respectively. And the mean dorsal mantle length (ML±SE) of the species were calculated as 12.71 ±0.64 cm. the comparison of growth slopes between males and females individuals did not provide evidence for significant differences between the sexes (Anova, p<0.05). Minimum body weight was 42.15 g, maximum 1452.60 g and the mean body weight was 405.59 ± 51.69 g calculated (Table 2). During the study, larger specimens were more frequent in the spring. Of these individuals, 14 were males and 14 were females. Dorsal mantle length and total weight relationships of O. macropus individuals were estimated for males, females and total (Table 3).
Sepia elegans Blainville, 1827 and Eledone cirrhosa (Lamarck, 1798): Only 1 specimen of S. elegans (Dorsal mantle length value was 4.2 cm, total length was 15.1 cm and weight was 4.68 g, a female individual) and E. cirrhosa (Dorsal mantle length 13.5 cm and weight 281.46 g, a male individual) caught.
Many studies have defined the cephalopod families and biology in the Mediterranean Sea. Mediterranean teuthofauna includes 65 species; about 9% of the world teuthofauna. Only 53 of them are represented as well established populations in the Mediterranean basin, all the others recently entered from the Atlantic Ocean and from the Red Sea through the Suez Chanel (Bello, 2003).
Indeed, recently many surveys were carried out in the eastern Mediterranean, namely in the north-western Mediterranean (Quetglas et al., 2000), western Mediterranean (Gonzalez and Sanchez, 2002), in Balearic Islands (Massuti and Renones, 2005), in Tyrrhenian Sea and Catalan Sea (Sanchez et al., 1998), in Libyan coast of Mediterranean (Rawag et al., 2004), Ionian (Lefkaditou et al., 2001 and Machias et al, 2001) and Aegean Sea (Tsepes et al., 1999; Salman et al, 2000; Tserpes and Peristeraki, 2002 and Salman and Katagan, 2004), which have shown the occurrence of many cephalopod species. Mediterranean Sea cephalopod fauna does not show the same biodiversity, abundance and condition all over. It can be easily understood from the latest studies that cephalopod fauna in the west includes 34 (Gonzalez and Sanchez, 2002), north western Mediterranean 30 (Quetglas et al., 2000), Tyrrhanian Sea 36 (Sanchez et al., 1998), Ionian Sea 24 (Tursi and D’Onghia, 1992) and Aegean Sea 38 species (Salman et al., 2002). The same situation has been shown in the Turkish seas, the cephalopod distribution (not only in the number of species but also fishery yield) shows differences among the Sea of Marmara, Aegean Sea and Mediterranean Sea and their coasts off Turkey (Salman and Katagan, 2004).
Therefore the theory “west-more-speciesthan- east” is in need of some adjustment according present cephalopod situation in Mediterranean Sea (Bello, 2003). And still no hypothesis has been given. As a matter of fact there is a comparable reduce of species and abundance from western Mediterranean to Eastern Mediterranean (Mangold and Boletzky, 1988 and Bello, 2003).
Studies on teuthofauna of cephalopod in Turkish water have been carried out since the study of Katagan and Kocatas (1990). A total of 43 species (i.e. 65% of Mediterranean teuthofauna) have been reported, comprising 11 species in the Sea of Marmara, 38 in the Aegean Sea and 24 in the Turkish coasts of Mediterranean Sea (Salman et al., 2002). The majority of those studies were carried out in the Aegean Sea (Katagan et al., 1992; Salman et al., 1997; Salman et al., 2000 and Salman et al., 2003). The same situation has been shown in the Aegean Sea; Salman et al. (1997) have determined the bathymetric distribution and catch composition in Aegean Sea and they divided the Aegean Sea into two parts; North and South Aegean. They found 27 cephalopod species from the northern and 16 cephalopod species from the southern Aegean Sea. Regional comparison of the northern and southern Aegean Sea showed that the CPUE was less in the southern Aegean Sea, the mean cephalopod catches per trawl were 4.48 kg/trawl/h in the northern Aegean and 3.46 kg/trawl/h in the southern Aegean found. The southern Aegean Sea is located around and near our study area. Compared with the data of Salman et al. (1997), lower numbers of cephalopod species were obtained in the present study. The differences between the number and abundance of the cephalopod species can also stem from the difference in research vessel, bottom trawl, larger geographical region and depths and also smaller mesh size codend gears used. So it is not possible to compare the catches between the regions accurately.
The similar situation about reducing the number of species and biodiversity had shown easily the Turkish coasts of Mediterranean Sea. i.e. Salman and Katagan (2004) separated the Mediterranean Sea into two parts; the Western Mediterranean and Eastern Mediterranean Sea. They found 21 Cephalopod species in the Western part, and 17 Cephalopod species for the Eastern part, compared with the number and abundance of cephalopod fauna that they found 5 species more than the present study at the same depth (in depth between 20 and 200 m). In this case, they used different mesh size bottom trawl (20 mm mesh size) and also used different depth between 20 and 500 m (generally they used higher depths from us). In this study hauls were made at 150 m on average. This made possible as well as the use of smaller mesh size in codend (22 versus 20 mm between knots).
Salman et al. (1999) found Octopus aegina between 60 and 70m depths in the southern coast of Turkey and they captured Octopoteuthis megaptera from the western coast at the first time. In our study O. aegina and O. megaptera specimens were not captured bebecause of using different seas and trawl gears (beam trawl versus bottom trawl) for O. megaptera and, different mesh size for O. aegina (22 versus 20 mm between knots).
Eledone cirrhosa and Rossia macrosoma were noticed for the first time from the middle part of Turkey’s Mediterranean coast by Salman et al., (2002). In the current study no Rossia macrosoma specimens were caught because this specie usually inhabits at depths below 200 m. On the other hand in the current study, only one Eledone cirrhosa specimen was captured. This is the first appearance of this species in Iskenderun Bay (Eastern Mediterranean coast of Turkey).
With that of our previous study (Duysak et al., 2004) in the Akkuyu Bay, which is western neighbor of our studying area, 7 cephalopod species were reported. In present study, Octopus defilippi and Octopus aegina specimens, which had been observed in the previous study, were not sampled. Meanwhile Duysak et al. (2004) could not find Illex coindetii and Eledone cirrhosa from the Akkuyu coast. When compared the size and sex ratio of cephalopod species between two studying areas, it was appreciated that no big differences between the two neighborhood studying areas were found.
Comparing the biological parameters of the cephalopod species studied, shown the similar situation with the western Mediterranean relatives (Nesis, 1982; Roper et al., 1984 and Guerra, 1992) and Aegean relatives in Turkish seas (Salman et al., 1997). The small differences like sex ratio, might be explain that caught different number of individuals in this study. And comparing with the Akkuyu Bay which is the western neighbor of our studying area, no big differences were found between the size and sex ratio (Duysak et al., 2004).
Conclusion
In this study, it was defined the species that are important for the fisheries and also for the cephalopod fauna and the biology of those species. It was defined 8 cephalopod species in this study. However, there might be uncertainty about the result. Turkey coasts to Eastern Mediterranean might be more cephalopod species. In this study, the fisherman’s fishing routes and fishing equipment were used. Maybe if the different nets (which equipped with different mesh size) and fishing equipment were used, at night or in deep sea, more species might have caught. As a result of this study it was define the species that are important for the fisheries and it also enlighten the future studies.
Acknowledgement
The authors would like to thank Mr. Peter Hubbert for constructive points for the paper and Dr. Jose Xavier for the helpful comments in many of the aspects the paper.
1631
References
- Anonymous, (2002). Impact assessment on fishing activities at Ceyhan Marine Terminal area ADANA. BTC Project Resetlement Action Plan Turkey Final Report. Annex 6.3: pp. 1-50
- Anonymous, (2004). Turkeys Statistical Yearbook. State institute of statistics, Prime Ministry Republic of Turkey, Ankara. 406.
- Bello, G. (2003). The biogeography of Mediterranean cephalopods. Biogeographia, 24: 209-226. Boyle, P.R., Daly, H. I. (2000). Fecundity and spawning in a deep-water cirromorph octopus. Marine Biology, 137: 317-324.
- Duysak, Ö., Türeli, C., Erdem, Ü. (2004). Akkuyu Koyu Cephalopod Faunasi (Eastern Mediterranean-Mersin, Turkey). Turkish Journal of Aquatic Life, 2: 181-192.
- Gaertner, J.C., Bertrand, J.A., Samani, D. Souplet, A. (2005). Spatio-Temporal Organization Patterns of Demersal Assemblages of The East Coast Of Corsica (Mediterranean Sea). Vie et Milieu, 55: 81-89.
- Gonzalez, M. Sanchez, P. (2002). Cephalopod Assemblages Caught by Trawling Along the Iberian Peninsula Mediterranean Coast. Scientia Marina, 66: 199-208.
- Guerra, A. (1992). Mollusca, Cephalopoda in Ramos, M. A. et al., eds, Fauna Iberica, Museo Nacional de Ciencias Naturales, CSIC, Vol. 1. 327 p., Madrid, Spain.
- Iyiduvar, O. (1986). Hydrografic Characteristics of Iskenderun Bay, M.Sc. Thesis, M.E.T.U. I.M.S., Erdemli, Içel.
- Kallianiotis, A., Sophronidis, K., Vidoris P., Tselepides, A. (2000). Demersal fish and megafaunal assemblages on the Cretan continental shelf and slope (NE Mediterranean): seasonal variation in species density, biomass and diversity. Progress in Oceanograph,. 46: 429–455.
- Katagan, T., Kocatas, A. (1990). Note preliminaire sur les Cephalopodes des eaux Turques. Rapp. Comm. Int. Mer Medit., 32 (1): 242.
- Katagan, T., Salman, A., Benli, H.A. (1992). Nouvelles Observations sur Ommastrephes bartrami (Lesueur, 1821) (Cephalopoda, Ommastrephidae) Dans Le Bassin Mediterraneen Oriental. Rapp. Comm. Int. Mer. Medit., 33: p. 298.
- Lefkaditou, E., Maiorano P., Mytilineou, C.H. (2001). Cephalopod Species Captured by Deep-water Exploratory Trawling in the Eastern Ionian Sea, Scientific Council Meeting – September 2001. Northwest Atlantic Fisheries Organization, 1, 131 p.
- Lloret, J., Lleonart, J. (2002). Recruitment Dynamics of Eight Fishery Species in the Northwestern Mediterranean Sea. Scientia Marina, 66: 77-82.
- Machias, A., Vassilopoulou, V., Vatsos, D., Bekas, P., Kallianiotis, A., Papaconstantinou, C., Tsimenides, N. (2001). Bottom Trawl Discards in the Northeastern Mediterranean Sea. Fisheries Resources, 53: 181-195.
- Mangold, K., Boletzky, S.V. (1987). Cephalopodes. Fiches FAO d’identification des escapes pour les besoins de la peche. (Revision 1) Mediterranee et Mer Noire, Zone de peche, 37 (1): 633-714.
- Mangold, K., Boletzky, S.V. (1988). Mediterranean Cephalopod Fauna. In: M.R. Clarke and E.R. Trueman eds, The Mollusca, 12, Paleontology and Neontology of Cephalopods, Acedemic Press 315-330, London and New York.
- Massuti, E., Renones, O. (2005). Demersal Research Assemblages in the Trawl Fishing grounds off the Balearic Islands (Western Mediterranean). Scientia Marina, 69 (1): 197-181.
- Nesis, K. N. (1987). Cephalopod of the world. Squids, Cuttlefishes, Octopuses and Allies, translated from Russian by B. S. Levitov.
- Nishiguchi, M.K. (2000). Temperature affects species distribution in symbiotic populations of Vibrio spp.. Applied and Environmental Microbiology, 66: 3550-3555.
- Norman, M.D. (2000). Cephalopods: a world guide, pp.320 IKAN Publishing, Frankfurt.
- Quetglas, A., Carbonell, A., Sanchez, P. (2000). Demersal Continental Shelf and Upper Slope Cephalopod Assemblages From The Balearic Sea (North-Western Mediterranean). Biological Aspects of Some Deep-Sea Species. Estuarine, Coastal and Shelf Science, 50: 739–749.
- Rawag, A.A., Haddoud, D.A., Zgozi, S.W. (2004). Commercial demersal marine species of Libya. MedSudMed Technical Documents No.2., 75-81.
- Roper, C.F.E., Sweeney, M.J., Nauen, C.E. (1984). Cephalopods of the world. Food and Agriculture Organization, Rome, Italy. Vol. 3: 277 pp.
- Salman, A., Katagan, T., Benli, H.A. (1997). Bottom trawl teuthofauna of the Aegean Sea. Archive of Fishery and Marine Research,. 45(2): 183-196.
- Salman, A., Katagan, T., Boletzky, S.V. (1999). New Cephalopod Molluscs in the Eastern Mediterranean: Previously Unnoted Species or Recent Migrants? Vie et Milieu, 49(1): 11-17.
- Salman, A., Katagan, T., Gücü, A.C. (2000). The Distribution and Fishing of two Mediterranean Eledone spp. (Octopoda: Cephalopoda) in the Aegean Sea. Turkish Journal of Zoology, 24: 165–171.
- Salman, A., Katagan, T., Benli, H.A. (2002). Cephalopod Fauna of the Eastern Mediterranean. Turkish Journal of Zoology, 26: 47-52.
- Salman, A., Katagan, T., Benli, H.A. (2003). Vertical Distribution and Abundance of Juvenile Cephalopods in the Aegean Sea. Scientia Marina, 67 (2): 167-176.
- Salman, A., Katagan, T. (2004). Fisheries Yield of Cephalopods at Turkish Seas. Turkish Journal of Aquatic Life, 2 (2): 25- 32.
- Sánchez, P., González, A.F., Jereb, P., Laptikhovsky, V.V., Mangold, K.M., Nigmatullin, Ch.M., Ragonese, S. (1998). Chapter 4: Illex coindetii. In: Rodhouse et.al., eds, FAO Fisheries Technical Paper: Squid recruitment dynamics. The genus Illex as a model, the commercial Illex species and influences on variability, 376: 59-76.
- Tursi, A., D’Onghia, G. (1992). Cephalopods of the Ionian Sea (Mediterranean Sea). Oebalia, 18: 25-43.
- Tsepes, G., Peristeraki, P., Potamias, G., Tsimenides, N. (1999). Species Distribution In The Southern Aegean Sea Based On Bottom-Trawl Surveys. Aquatic Living Resources, 12 (3): 167-175.
- Tserpes, G., Peristeraki P. (2002). Trends in the Abundance of Demersal Species in the Southern Aegean Sea. Scientia Marina, 66: 243-252.










