Keywords
Bacterial disease, Onchorhynchus mykiss, Turkey
Özet
Türkiye’de Gökku?a?? Alabal??? (Oncorhynchus mykiss Walbaum, 1792)’nda Görülen Bakteriyel Hastal?klar?n Tespiti ve Antibiyotik Duyarl?l?klar?n?n Belirlenmesi
Bu çal??mada Türkiye iç sular?nda ve kafeslerde yeti?tiricili?i yap?lan gökku?a?? alabal?klar?n-daki bakteriyel patojen etkenler ara?t?r?lm??t?r. Bu kapsamda 2005–2008 y?llar? aras?nda Türki-ye’ nin farkl? lokalitelerinden temin edilen ve bakteriyel hastal?k ?üphesi gösteren 20 alabal?k populasyonuna ait 692 adet materyalden etken izolasyonu yap?lm??t?r. ?detifikasyon çal??malar? sonucunda 11 farkl? bakteriyel patojen etken izole edilmi?tir. Bunlar Acinetobacter sp., Aer-omonas hydrophila, A. sobria, Citrobacter freundii, Cytophaga psychrophila, Lactococcus garvieae, Micrococcus sp., Streptococcus iniae, Vibrio alginolyticus, V. anguillarum, V. salmonicida ve Yersinia ruckeri türleri olup en s?k izole edilen etken Yersinia ruckeri’dir. Yap?lan antibiyogram testleri sonucunda enroflaksasinin, oksitetrasiklin ve florfenikol elde edilen etkenlere kar?? etkili oldu?u belirlenmi?tir.
Anahtar Kelimeler
Bakteriyel hastal?k, Onchorhynchus mykiss, Türkiye
Introduction
In Turkey, fish culture began with rainbow trout farming in concrete and earthen ponds in 1960’s. In 1980 the rainbow trout production that was 3.000 tonnes, reached 85.000 tons in 2010 (Celikkale et al., 1998; TUIK, 2010).
Rainbow trout, Oncorhynchus mykiss, one of the most important fish species for freshwater aquaculture in Turkey also cultured in sea cages in the Black Sea since the early 1990s. The most significant factor affecting their culture is the in-cidence of microbial pathology. The major mi-crobial diseases in freshwater farms are: furuncu-losis, caused by Aeromonas salmonicida; enteric redmouth disease by Yersinia ruckeri, and infec-tions with Flavobacterium psychrophilum. These diseases are also common worldwide and pro-duce considerable economic losses in the fish farming industry (Austin and Austin, 2007).
Economic consequences can be reduced by decreased mortality and treatment with appropri-ate antibiotics. Antibacterial treatments are most often administered to fish through drug supple-mented feeds. Contamination of the surrounding environment by these drugs occurs principally through uneaten feed, and feces (Hirsch et al., 1999). In addition the use of inappropriate antibi-otics can in some cases lead to the development of antimicrobial resistance in fish pathogens and environmental bacteria (Alderman and Hastings, 1999).
The aims of this study were to isolate and identify pathogenic fish bacteria from rainbow trout farms in Turkey and determine the most ef-fective antibiotic treatment against them.
Materials and Methods
Fish samples
Diseased rainbow trout were brought from freshwater fish farms in different cities and cul-tured in sea cages between 2005-2008. Fish were sampled from 20 farms. During the four years, the samples usually consisted of six fish per farm per season. Fish were also sampled during dis-ease outbreaks. In total 692 infected samples from Persembe (Ordu), Yomra (Trabzon), Kozan (Adana), Fethiye (Mugla, 4 different localities), Kemalpasa (Izmir), Catak (Van), Hassa (Hatay), Banaz (Usak), Trabzon, Mudurnu (Bolu), Nazilli (Ayd?n), Akyaz? (Adapazar?), Bucak (Burdur, 2 different localities), Dalaman (Mugla), Keles (Bursa), Almus (Tokat) were received. All fish samples were investigated at Bornova Veterinary Control and Research Institute in Izmir.
Identification of bacteria
The 692 fish (5-300 g) were transported to the laboratory on ice and dissected for bacteriologi-cal examination. All were examined externally and internally. Samples from spleen, skin, kidney and liver of all fish from each outbreak were streaked onto the following standard selective media; Tryptic soy agar (TSA), Brain Heart Infu-sion Agar (BHI), Blood Agar (BA), Thiosulfate-Citrate-Bile Salt Sucrose Agar (TCBS), Shotts-Watman Medium Agar (WS) and Kidney Disease Medium Agar (KDM). All the inoculated media were incubated at 20-22?C for 24±48 h. F. psy-chrophilum colonies that grew on CA at 15 ºC for 3-4 days. Bacterial strains were examined by phenotypic tests. Identification was carried out by conventional biochemical tests as described by Buchanan et al., (2001) and Austin and Austin (2007).
Colonies were examined morphologically ac-cording to shape, color and bacterial motility. These were characterized biochemically with the following tests: Gram staining, cytochrome-oxidase, oxidative/fermentative test, catalase, O/129, ß-galactosidase, arginine dihydrolase, ly-sine decarboxylase, ornithine decarboxylase, cit-rate utilization, H2S production, urease, indole production, methyl red, voges proskauer, gelati-nase, fermentation of glucose, mannitol, inositol, sorbitol, rhamnose, saccharose, amygdalin, arab-inose and lactose.
Antimicrobial susceptibility tests
Antibiotic susceptibility test was determined by the disc diffusion method (Dalsgaard, 2001). Acinetobacter sp., Aeromonas hydrophila, Aer-omonas sobria, Citrobacter freundii, Lactococ-cus garvieae, Micrococcus luteus, Streptococcus iniae, Vibrio alginolyticus, Vibrio anguillarum, and Yersinia ruckeri were tested on Mueller-Hinton Agar incubated at 22 ºC overnight. Fla-vobacterium psychrophilum was tested on CA, incubated at 15 ºC and the zones read 3-4 days. Disks containing the following antibiotics were used; enrofloxacin (5 μg), oxytetracycline (30μg), trimethoprim-sulphamethoxazole (25μg), flumequin (30μg), doxycycline (5μg), oxolinic acid (2 μg) and florfenikol (30μg).
Results and Discussion
Eleven different species of bacteria were iso-lated from 692 infected fish. Biochemical charac-teristics of the isolates are shown in Table 1 and Table 2. Isolated pathogens and regions were shown in Table 3. The geographical distribution of the pathogens has been shown in Figure 1. Comparasion between the frequencies of isola-tion of the different bacterial groups detected in survey of bacteria isolated from isolated farmed rainbow trout in Table 4. From skin and the in-ternal organs of the fish, strains belonging to Aci-netobacter sp., A. hydrophila, A. sobria, C. freundii, F. psychrophilum, L. garvieae, M. lute-us, S. iniae, V. alginolyticus, V. anguillarum and Y. ruckeri were isolated.
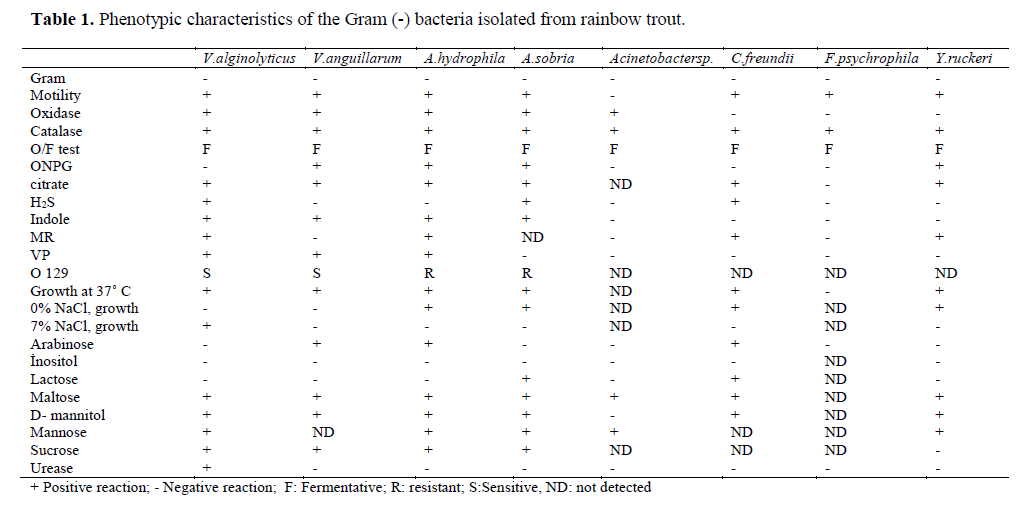
Table 1. Phenotypic characteristics of the Gram (-) bacteria isolated from rainbow trout.
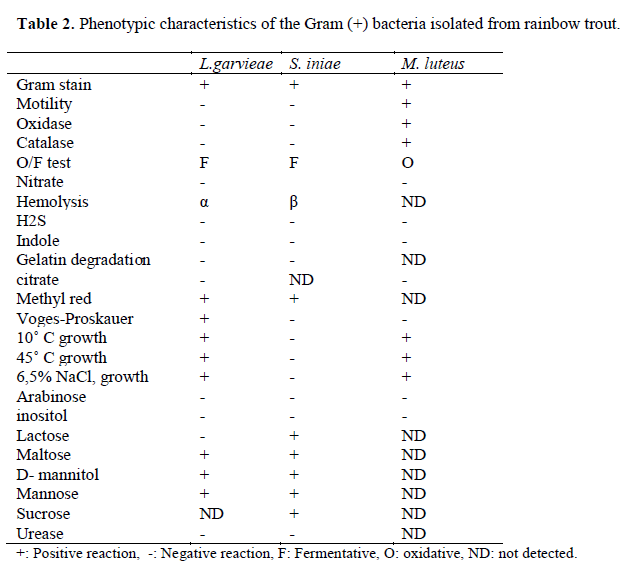
Table 2. Phenotypic characteristics of the Gram (+) bacteria isolated from rainbow trout.

Table 3. Regions isolated pathogens
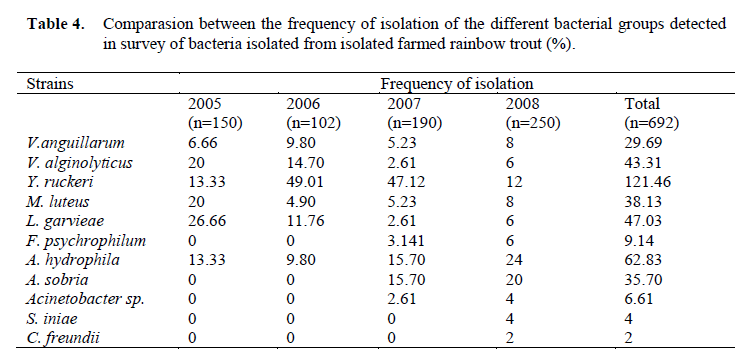
Table 4. Comparasion between the frequency of isolation of the different bacterial groups detected in survey of bacteria isolated from isolated farmed rainbow trout (%).
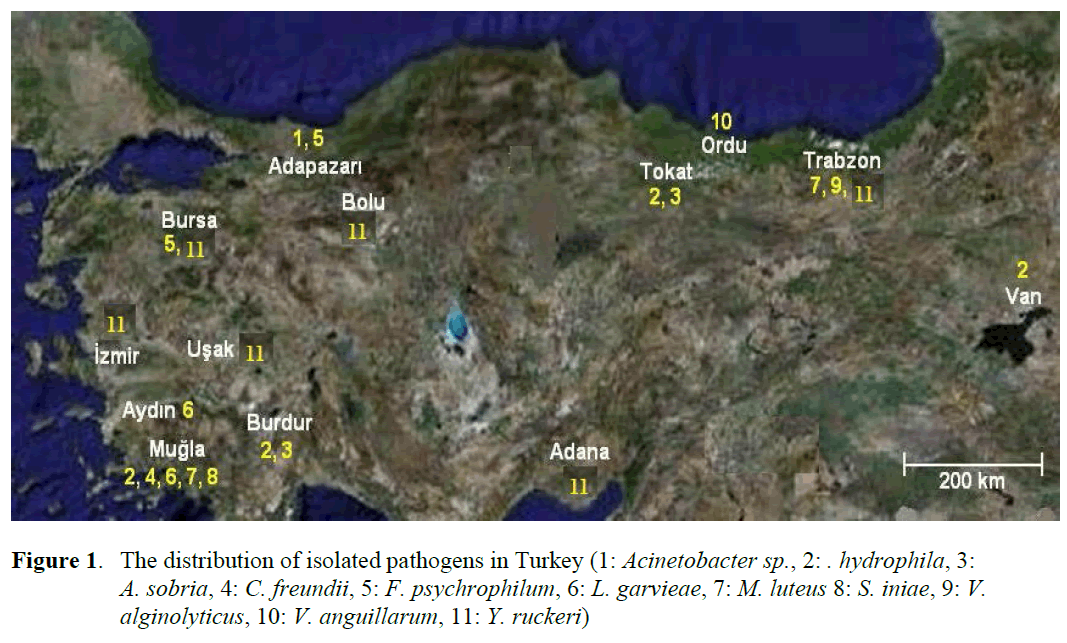
Figure 1. The distribution of isolated pathogens in Turkey (1: Acinetobacter sp., 2: . hydrophila, 3: A. sobria, 4: C. freundii, 5: F. psychrophilum, 6: L. garvieae, 7: M. luteus 8: S. iniae, 9: V. alginolyticus, 10: V. anguillarum, 11: Y. ruckeri)
The most common pathogen isolated from rainbow trout freshwater farms were Y. ruckeri. Y. ruckeri was isolated from the liver, spleen, and kidney. A. hydrophila, L. garvieae, V. anguilla-rum were isolated from kidney, liver, spleen, and skin lesions. When fish has clinical signs of dis-ease, the following bacteria were isolated; A. so-bria. A. hydrophila, F. psychrophilum (dark pig-mentation, fried fin), Y. ruckeri (hemorrhage on liver, enlarged spleen, and exophthalmia), C. fre-undii (ulcerative lesions on skin, pale liver, surfa-ce haemorrhages), M. luteus (haemorrhages, ex-ophhalmia), S. iniae (discoloration, bilaterial ex-ophthalmia, corneal opacity, haemorrhaging in the eye), L. garvieae (exophthalmia, darkening skin, haemorrhagic enteritis), V.alginolyticus (bacterial septicaemia, darkening skin) and V. anguillarum (haemorrhage in anal and pectoral fins, haemorrhage in liver). Antibiotic suscepti-bilities of isolated pathogens were given Table 5. The isolates were found susceptible to enfroflox-acin, oxytetracycline and florfenicol.
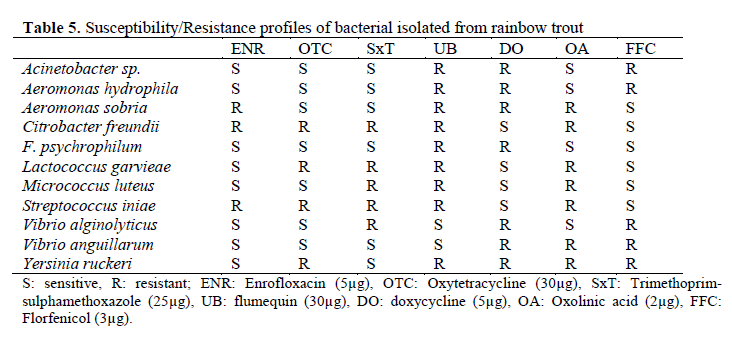
Table 5. Susceptibility/Resistance profiles of bacterial isolated from rainbow trout
In this study, 692 infected rainbow trout were investigated. As a result of the bacterial samples skin and from internal organs of the fish, Aci-netobacter sp., A. hydrophila, A. sobria, C. freundii, F. psychrophilum, L. garvieae, M. lute-us, S. iniae, V. alginolyticus, V. anguillarum, and Y. ruckeri were isolated. The most frequently iso-lated strain was identified as Yersinia ruckeri (Table 4). The presence of these bacteria both in aquatic environment and the internal fish organs have been often observed (Toranzo et al., 1993; Karata? et al., 2004; Ak?it and Kum, 2008).
Previous investigations on bacterial infections in Turkish marine and freshwater rainbow trout hatcheries have focused on disease outbreaks from a single pathogen Y. ruckeri (Timur and Timur, 1991; Karata? et al., 2004; Ak?it and Kum, 2008; Kay?? et al., 2009). To date, other than Y. ruckeri reported bacterial pathogens of rainbow trout in Turkey are Acinetobacter sp. (Yonar et al., 2010), A. hydrophila (Kirkan, et al., 2003; Kay?? et al., 2009), A. sobria (Kay?? et al., 2009), C. freundii (Ayd?n et al.,1997), F. psy-chrophilum (Gültepe et al., 2006; Kay?? et al., 2009), L. garvieae (Ak?it and Kum, 2008; Diler et al., 2002), M. luteus (Ayd?n et al., 2005), S. in-iae (Ayd?n et al., 2005), V. anguillarum (Tanr?-kul, 2007; Ak?it and Kum, 2008). All these agents were isolated in this study too. V. algino- lyticus was isolated from rainbow trout cultured in the sea cages, although it was not reported in previous investigations Ak?it and Kum, 2008; Kay?? et al., 2009.
In this study antibiotic susceptibility tests re-vealed that Acinetobacter sp. strains were sensi-tive to enrofloxacin, oxytetracycline, trime-thoprim-sulphamethoxazole and oxolinic acid. On the other hand, some of strains were found to be resistant to flumequin and doxycycline. Previ-ous reports were indicated Acinetobacter sp. strains were sensitivity oxytetracycline (Austin and Austin, 2007). Aeromonas spp. is adapted for antimicrobial resistance monitoring because of its natural susceptibility to main antimicrobial drugs used in aquaculture (Spanggard et al., 1993), par-ticularly to trimethoprim-sulphamethoxazole, oxytetracycline, quinolones (oxolinic acid and flumequine) and florfenicol. In this study, A. hy-drophila strains were sensitivity to enrofloxacin, oxytetracycline,trimethoprim-sulphamethoxazole and oxolinic acid as predicted. A. sobria strains were sensitivity to oxytetracycline, trimethoprim-sulphamethoxazole and florfenical as predicted. On the other hand, some of strains were found to be resistant to enrofloxacin and doxycycline.
According to Baya et al. (1990), C. freundii strains were resistant to chloramphenicol, poten-tiated sulphonamides and tetracycline. In this study, the strains were sensitive to doxycycline and florfenicol. Oxytetracycline and oxolinic acid are considered to be the antibiotic of choice for control of infection by F. psychrophilum (Holt et al., 1993). Our antibiotic susceptibility tests re-vealed that all of the pathogens isolates were sus-ceptible to enrofloxacin, oxytetracycline, trime-thoprim-sulphamethoxazole, oxolinic acid and florfenicol. According to the results of this study, L. garvieae isolates were resistant to oxytetracyc-line, trimethoprim-sulphamethoxazole, flumequin and oxolinic acid and susceptible to enrofloxacin, doxycycline and florfenicol. When the other stu-dies were evaluated, it was determined that L. garvieae was susceptible to amphenicoles, oxytetracyclineand novobiocin. Tetracycline, and chloramphenicol susceptibilities were also repor-ted (Diler et al., 2002). These different results may be due to the differences of L. garvieae stra-ins and antibiotics usage in the area.
M. luteus was resistant to potentiated sul-phanamides and oxytetracycline which contrast to the findings of Austin and Stobie (1992). Ac-cording to the results of present study enrofloxa-cin, oxytetracycline, doxycycline and florfenicol have been observed to effective to treat fish in-fected M. luteus. S.iniae isolates was susceptible to oxytetracycline (Darwish and Ismail, 2003) for the control of the infection. S.iniae was sensitive to doxycycline and florfenicol in the present study. V. alginolyticus strains were sensitive to enrofloxacin, oxytetracycline, flumequin and ox-olinic acid as predicted. On the other hand, some of strains were found to be resistant to trimethop-rim-sulphamethoxazol, doxycycline and florfenicol. Previous reports indicated that V. al-ginolyticus was sensitive to chloramphenicol and nitrofurantoin (Report, 1969). According to an-timicrobial susceptibility test in this study, V. an-guillarum isolates were found to be sensitive to enrofloxacin, oxytetracycline, trimethoprim-sulphamethoxazole and florfenicol. Y. ruckeri strains were sensitive to enrofloxacin and trime-thoprim-sulphamethoxazole in present study. These susceptibility results were similar to Kara-tas et al., (1990) and Savvidis (2004).
Conclusion
In conclusion, the present study Gram nega-tive and Gram positive bacteria were identified at different areas in Turkey. The results in the pre-sent study show that it is necessary to carry out extensive monitoring of fish pathogenic bacteria, in order to obtain more knowledge of the most important diseases which occur in different rain-bow trout farm in Turkey. The knowledge of the distribution of bacterial pathogens and a subse-quent investigation of the characteristics of the bacteria is necessary to evaluate which factors are important in establishing an infection. Also, anti-bacterial susceptibility test should be performed in consideration of antibacterial drugs which are used in aquaculture medicine in Turkey.
Acknowledgements
We want to special thanks to Dr. M. Didem Ercan for precious assistance to prepare this pa-per.
426
References
- nAksit, D., Kum, C., (2008). Determination of An-tibiotics Susceptibility of Frequently Isolat-ed Pathogen Microorganisms from Rainbow trout (Oncorhynchus mykiss Walbaum 1792), The Journal of the University of Yuzuncu Yil, Faculty of Veterinary Medi-cine, 19(1): 1-7
- nAlderman, D.J., Hastings, T.S., (1998). Antibi-otic use in aquaculture: development of an-tibiotic resistance potential for consumer health risks, International Journal of Food Science Technology, 33: 139-155. doi: 10.1046/j.1365-2621.1998.3320139.x
- nAustin, B., Austin, D.A., (2007). Bacterial Fish Pathogens Diseases of Farmed and Wild Fish, 4th edt., Springer-Verlag, Praxis Pub-lishing, UK, 552p
- nAustin, B., Stobie, M., (1992). Recovery of Mi-crococcus luteus and presumptive Plano-coccus sp. from moribund fish during out-breaks of rainbow trout (Oncorhynchus mykiss Walbaum) fry syndrome (RTFS) inEngland, Journal of Fish Diseases, 15: 203-206.doi: 10.1111/j.1365-2761.1992.tb00655.x
- nAydın, S., Celebi, S., Akyurt, İ., (1997). Clinical and pathological investigation of Citrobacter freundii infection in rainbow trout (Oncorhynchus mykiss Walbaum), Turkish Journal of Veterinary Animal Sci-ences, 21(6): 497-501
- nAydın, S., Ciltas, A., Yetim, H., Akyurt, I., (2005). Clinical, pathological and haemato-logical effects of Micrococcus luteus infec-tions in rainbow trout (Oncorhynchus mykiss Walbaum), Journal of Animal and Veteri-nary Advances, 4(2): 167-174
- nBaya, A.M., Lupiani, B., Hetrick, F.M., Toranzo, A.E., (1990). Increasing importance of Citrobacter freundii as a fish pathogen, Fish Health Section/American Fisheries Society Newsletter, 18: 4
- nBuchanan, R.E., Gibbons, N.E., (1974). Bergey’s Manual of Determinative Bacteriology, Eighth Edition, The Williams, Wilkins Company/Baltimore
- nCelikkale, S.M., Okumus, I., Kurtoglu, I.Z., Bascinar, N., (1998). The present and poten-tial of coastal aquaculture in the black sea, First International Symposioum on Fisheries and Ecology Proceedings, 02-04 September, Trabzon
- nDalsgaard, I., (2001). Selection of media for an-timicrobial susceptibility testing of fish pathogenic bacteria, Aquaculture, 196: 267-275.doi: 10.1016/S0044-8486(01)00538-5
- nDarwish, A.M., Ismaiel, A.A., (2003). Laborato-ry efficacy of amoxicillin for the control of Streptococccus iniae infection in sunshine bass, Journal of Aquatic Animal Health, 15: 209-214. doi: 10.1577/H03-016
- nDiler, Ö., Altun, S., Adiloglu, A.K., Kubilay, A., Isıklı, B., (2002). First occurrence of Strep-toccosis affecting farmed rainbow trout in Turkey, European Association of Fish Path-alogists, 22: 21-26
- nGultepe, N., Tanrıkul, T.T., (2006). Treatment methods of Flavobacterium psychrophilum: Cause of rainbow trout fry syndrome(RTSF) and bacterial cold-water disease (BCWD) in Turkey, Journal of Fisheries In-ternational, 2(4): 102-105
- nHirsch, R., Ternes, T., Haberer, K., Kratz, K.L., (1999). Occurrence of antibiotics in the aquatic environment, Science of The Total Environment, 225: 109-118.doi: 10.1016/S0048-9697(98)00337-4
- nHolt, R.A., Rohove, J.S., Fryer, J.L., (1993). Bac-terial cold water disease, in Inglis et al., eds, Bacterial cold water disease, Blackwell Sci-entific Publications, UK, 3-23
- nKaratas, S., Candan, A., Demircan, D., (2004). Enteric red mouth disease in cultured rain-bow trout (Oncorhynchus mykiss) on the black sea coast of Turkey, The Israeli Jour-nal of Aquaculture-Bamidgeh, 56(3): 226-231
- nKayıs, S., Capkın, E., Balta, F., Altinok, I., (2009). Bacteria in Rainbow Trout (On-corhynchus mykiss) in the Southern Black Sea Region of Turkey - A Survey, The Is-raeli Journal of Aquaculture-Bamidgeh, 61(4): 339-344
- nKirkan, S., Goksoy, E.O., Kaya, O., (2003) Isola-tion and antimicrobial susceptibility of Aer-omonas salmonicida in rainbow trout (On-corhynchus mykiss) in Turkey hatchery farms, Journal of Veterinary Medicine, Se-ries B, 50: 339-342.doi: 10.1046/j.1439-0450.2003.00671.x
- nReport, (1969). Joint Committee on the Use of Antibiotics in Animal Husbandry and Veter-inary Medicine (Swann Report), London, H.M.S.O
- nSavvidis G.K., (1990). First isolation of Yersinia ruckeri from rainbow trout, Oncorhynchus mykiss, in Greece, European Association of Fish Pathalogists, 10(5): 131
- nSpanggard, B., Jorgensen, F., Gram, L., Huss, H.H., (1993). Antibiotic- Resistance in Bac-teria Isolated from 3 Fresh-Water Fish Farms and an Unpolluted Stream in Den-mark, Aquaculture, 115: 195-207.doi: 10.1016/0044-8486(93)90136-M
- nTanrıkul, T.T., (2007). Vibriosis as an epizootic disease of of rainbow trout (Oncorhynchus mykiss) in Turkey, Pakistan Journal of Bio-logical Sciences, 10: 1733-1737.doi: 10.3923/pjbs2007.1733.1737
- nTimur, G., Timur, M., (1991). An outbreak of en-teric red mouth disease in farmed rainbow trout (O. mykiss) in Turkey, European Asso-ciation of Fish Pathalogists, 7(1): 18
- nTUIK., (2010). Fisheries Production Statistics for 2009, Ankara, Turkey
- nToranzo, A. E., Novoa, B., Romalde, J.L., Núñez, S., Devesa, S., Mariño, E. Silva, R., Martínez, E., Figueras, A., Barja, J.L.,(1993). Microflora associated with healthy and diseased turbot (Scophthalmus maxi-mus) from three farms in Northwest Spain, Aquaculture, 114: 189-202.doi: 10.1016/0044-8486(93)90295-A
- nYonar, M.E., Karahan, M., Kan, N.İ., Yonar, S., Saglam, N., (2010). Kahramanmaraş Bölge-sindeki Bazı Gökkuşağı Alabalığı (On-corhynchus mykiss) İşletmelerinde Görülen Acinetobacter Sp. Enfeksiyonunun Araştırılması, Jornal of FisheriesScienc-es.com, 4: 287-293.doi:10.3153/jfscom.201003












