Keywords
Black Sea turbot, Psetta maxima, Egg, Disinfection, Iodine
Özet: ?yot Uygulamas?n?n Karadeniz Kalkan Bal???, Psetta maxima Yumurtalar?n?n Ç?k?? Oran?na Etkisi
Dezenfeksiyon amaçl? iyot uygulamas?n?n kalkan yumurtalar?n?n ç?k?? ba?ar?s? üzerine etkilerini ara?t?ran bir çal??ma yürütülmü?tür. Yumurtalar 100 500, 1500 ve 3000 ppm iyot çözeltisi içinde 10 dakika süre ile muamele edildi. En dü?ük ç?k?? oran? 3000 ppm iyot uygulanan yumurta grubunda (%70,0 ± 6,08) görüldü. Ç?k?? oran? kontrol grubunda (%97.6 ± 1.42) ve 100 ppm iyot ile muamele edilen grupta (%91.3 ± 4.04) di?er gruplardan önemli derecede yüksek olmu?tur (p <0.05). 1500 ve 3000 ppm iyot ile dezenfekte edilen yumurtalardan ç?kan larvalarda görülen anormallik oranlar? kontrol grubundan önemli oranda fazlad?r (p <0.05). S?f?r günlük, 1 günlük ve 3 günlük yumurta gruplar? on dakika süre ile 100 ppm iyot çözeltisine maruz b?rak?lm??t?r. 1 ve 3 günlük yumurtalar?ndan ç?kan larvalarda görülen anormallikler s?f?r günlük (morula a?amas?ndaki) yumurta grubundan önemli oranda fazlad?r (p <0.05). Sonuç olarak, kalkan yumurta dezenfeksiyonu için morula a?amas?nda 100 ppm iyot uygulamas? uygun olabilir.
Anahtar Kelimeler: Karadeniz kalkan?, Psetta maxima, Yumurta, Desenfeksiyon, ?yod
Introduction
Turbot has been identified as a suitable species for aquaculture in Turkey but production of sufficient juvenile fish is a limiting factor in development of the industry. This species have been reported to be susceptible to, or they may carry, serious fish pathogens including Vibriosis, Streptococcosis, Flexibacteriosis, viral hemorrhagic septicemia, Trichodiniasis, Dactylogyrosis, Scuticociliatosis, Saprolegnia sp. (Sava? and Türe, 2007).
In a marine fish farm, the control of animal health is a major factor of productivity. The practice of egg incubation under intensive conditions increases the risk of microbial proliferation. It is also important to prevent transmission of bacterial diseases from broodstock to eggs. The quality of eggs and larvae produced in hatcheries are considered an important limiting factor in fry production (Kjørsvik et al., 1990) and, consequently, in the development of the aquaculture industry (Bromage, 1995).
Mass mortalities due to infectious and noninfectious diseases have often occurred in larvae reared in hatcheries (Muroga, 1995). Schachte (1979) reported that bacteria may be responsible for some mortality of fish eggs incubated in hatchery tanks. According to Barker et al. (1989) and Sauter et al. (1987), bacteria can influence egg survival. For this reason, disinfection of eggs has been widely used to reduce egg mortality and improve rearing success during the yolk sac and first feeding stages (Salvesen et al., 1997; Harboe et al., 1994; Bergh and Jelmert, 1996; Peck et al., 2004). A number of methods have been developed to disinfect fish eggs. These methods include using solutions of formalin, hydrogen peroxide, iodine, methylene blue, ozone, benzalconium chloride or sodium hypochlorite. Many of these solutions are highly effective against gram-positive and gram-negative bacteria and fungi.
Iodine, one of the disinfectants has been shown to have relatively little toxicity to eggs of several fishes but is highly toxic to fish pathogens such as bacteria (McFadden, 1969) and viruses (Goldes and Mead, 1995; Batts et al., 1991). Therefore, especially in salmonids, iodine disinfection of eggs has been developed and applied widely. However, there have been few studies on the use of iodine to disinfect marine fish eggs (Hirazawa et al., 1999).
The aim of this study is to establish the appropriate egg developmental stage as well as the concentration for the iodine disinfection of turbot eggs.
Materials and Methods
Experimental trials were conducted at the Central Fisheries Research Institute (CFRI), Trabzon, Turkey. Eggs were collected from four years old hatchery-reared turbot by stripping and artificially fertilized. Eggs were fertilized in seawater by gently mixing with newly stripped milt from two males. Sperm quality was checked according to Suquet et al., (1995). Eggs were incubated in a 100 L tank supplied with seawater until disinfection. The temperature of the seawater was kept at 14±0.5ºC during experiments. Sand and 1-μm cartridge filtered and UV irradiated seawater of salinity of about 18‰ was used in the experiments.
To determine the effect of iodine treatment dose on the hatching rate, 10 g eggs (900 eggs g-1) were placed in aerated 1000 ml beakers with the following concentrations 100 ppm, 500 ppm, 1500 ppm and 3000 ppm of 10% PVP-iodine solution (Aqua-iodine: Argent Chemical Laboratory, USA). The eggs were soaked in the iodine for ten minutes and rinsed with running seawater for 45 seconds to remove the disinfectant. For the control (0 ppm iodine), the eggs were rinsed with seawater as in the disinfected eggs. About 100 individual floating eggs were transferred in to 0.5 mg l-1 penicillin G containing 1000 ml glass beakers. The beakers were kept in an incubator (Sanyo MIR 153 Sanyo Electric, Osaka, Japan) at 14 ºC.
The eggs were incubated under dim light to observe hatchability and survival until the end of the yolk-sac period. Dead eggs/embryos were removed and counted daily during the incubation period and the total number of hatched larvae registered at the end of the hatching period. The percentage of hatched larvae was determined in relation to the initial number of incubated eggs. There were three replicates for each treatment.
To determine the effect of iodine treatment time, 0-day-old eggs (early morula stage, 6 hrs after fertilization), 1-day-old (gastrula stage) eggs and 3-day-old (embryo begins to move) eggs were treated with a dose of 100 ppm iodine for ten minutes.
All analyses were performed with the program Statistica 7.0 for windows (Stat Soft, 1984-2004). To assess normality of distributions, a Kolmogorov–Smirnov test was used, and homogeneity of variances was tested using the Levene’s F-test (Zar 1999). One-way ANOVA was applied, followed by Tukey test to locate any differences among treatments. Percent data were performed with arcsine transformation. A significance level of 5% was used in all tests.
Results and Discussion
Table 1 shows hatching rate and abnormality of turbot eggs disinfected with different iodine concentrations for 10 minutes. The hatching percentage ranged from 70.0 to 97.6%. The treatment with 3000 ppm iodine presented lower hatching percentage (70.0 ±6.08%). Both the control group (97.6 ±1.42%) and the group disinfected with 100 ppm iodine (91.3 ±4.04%) resulted in slightly higher hatching percentage, and significantly different from the other disinfected groups (P < 0.05). As shown in Table 1 treatment of eggs with iodine significantly affected the egg hatch. However, there were no significant differences among the disinfected groups at 500, 1500 and 3000 ppm in hatching rates.
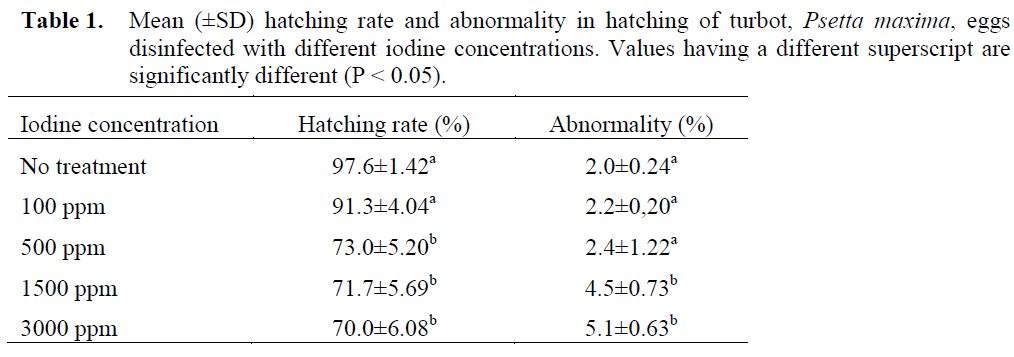
Table 1. Mean (±SD) hatching rate and abnormality in hatching of turbot, Psetta maxima, eggs disinfected with different iodine concentrations. Values having a different superscript are significantly different (P < 0.05).
Abnormalities at the hatching were low and varied between 2.0±0.24 and 5.1±0.63%. Only 1500 and 3000 ppm iodine treated groups were significantly different from the control group (P < 0.05).
The hatching rates and abnormalities varied between 82.7–86.0%, and 8.2–15.6%, respectively (Table 2) in the experiment for the effect of iodine treatment time. The hatching rates in all groups were similar, but differences in abnormalities between 0 day-old eggs and 1 and 3 day-old eggs were statistically significant (P < 0.05).
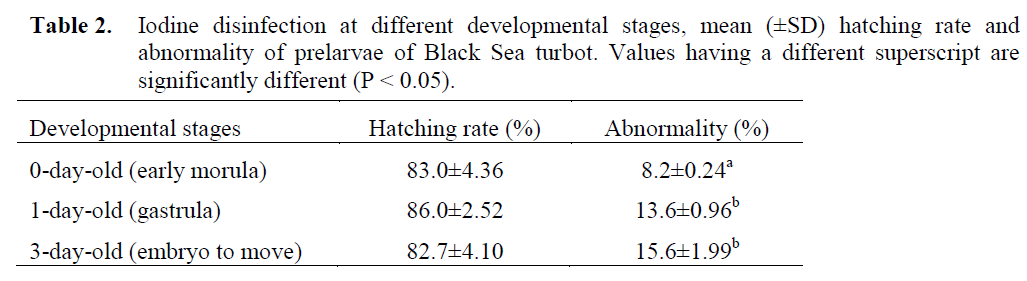
Table 2. Iodine disinfection at different developmental stages, mean (±SD) hatching rate and abnormality of prelarvae of Black Sea turbot. Values having a different superscript are significantly different (P < 0.05).
To investigate the tolerance of turbot eggs for iodine, eggs at the early morula stage were treated with 100-3000 ppm iodine for 10 minutes. The results show that concentrations of iodine ranging from 100 to 3000 ppm gave hatching percentages of over 70%, which were lower than the results of the control (0 ppm). The highest percent hatch (97.6%) was achieved at the control group. The treatment with iodine reduced the hatching rates, in contrast, increased abnormality significantly. Percent hatch was considerably lowered at higher iodine concentrations (> 100 ppm). This could imply that the high concentrations (> 100 ppm) had a toxic effect on the turbot eggs. Iodophores have low toxicity to fish eggs and are recommended as virucidal agents in fish hatcheries (MacFadden, 1969; Amend and Pietsch, 1972). It has been shown to have little toxicity to eggs of several fish but is toxic to fish pathogens. However, there are also reports on the toxicity of iodophor to fish eggs (Fowler and Banks, 1991). Toxic effects have previously been observed after 10-15 min treatments with ≥ 200 mg active iodine (McFadden, 1969; Wright and Snow, 1975; Subasinghe and Sommerville, 1985). Salvesen et al. (1991) reported that doses of Buffodine (iodophor: 1.06% free iodine) that reduced the bacterial load were above the toxic level. However, Hirazawa et al. (1999) reported that no bacterial growth was recognized in spotted halibut (Verasper variegatus) eggs treated with 75 ppm iodide for 15 min and the hatching rate were not significantly different from the control. The same study reported that no bacterial growth was observed in red sea bream (Pagrus major) egg treated with 100 and 200 ppm active iodide for 5 min and there is also no significant difference in the hatching rate of treated eggs and the control. Tendencia (2001) reported that at 15 and 20 ppm iodine, total bacterial count of grouper egg at the cleavage and eyed stages are significantly reduced; however, the hatching rate is also significantly lowered at these concentrations. Fowler and Banks (1990) report that iodophor disinfection (75 ppm for 30 min) of chinook eggs at the water hardening stage, resulted in 2-3% higher mortality at the eyed egg stage than untreated controls. Subsequent work by these authors demonstrated that a 30 min treatment at 50 ppm did not cause significant mortality (Fowler and Banks, 1991). Alderman (1984) concluded that 10 min treatments at 100 ppm iodine did not cause significant egg mortality in rainbow trout. The same author indicated that eggs from different broodstock have different degree of tolerance in iodine disinfection. According to Hirazawa et al. (1999) the optimum conditions for iodine disinfection of eggs vary among different species and should be determined separately for each fish species. Physiological and structural differences among
fish eggs are large, thus tolerance towards disinfectants must be variable (Davenport et al. 1986; Lønning et al. 1988). A concentration of 100 ppm iodine may therefore be more appropriate for adequate disinfection of turbot eggs.
To determine the effect of iodine treatment time, 0-day-old eggs (early morula stage, 6 hrs after fertilization), 1-day-old (gastrula stage) eggs and 3-day-old (embryo begins to move) eggs were treated with a dose of 100 ppm iodine for ten minutes. The hatching rates of eggs at all developmental stages examined were not influenced by iodine treatment. All stages can be considered suitable for disinfection. In consideration of abnormalities, the morula stage was most suitable among the three stages. In marine fish eggs, sensitivity at different developmental stages to iodophor had been investigated in Atlantic halibut by Bergh and Jelmert (1996) who suggested that 45 day-degrees were the most suitable for disinfection.
Hirazawa et al. (1999) reported that the most suitable stage for treatment was morula stage in Pagrus major and heart beating stage in Verasper variegatus. Tendencia (2003) showed that the best stage to disinfect grouper, Epinephelus coioides, egg is at the late neurula stage where the embryo shows twitching movement. The results obtained in this study are in agreement with the observations of Hirazawa et al. (1999) in red sea bream eggs, but in contrast with the findings of Bergh and Jelmert (1996) in Atlantic halibut eggs and Tendencia (2003) in grouper eggs. It is important to note that there might be a difference between species in available range of developmental stages (Hirazawa et al., 1999). Different fish species and different egg stages have different tolerance to iodine. Therefore it is important to establish the developmental stage and iodine concentration and exposure time that are safe and effective in reducing the bacterial load and at the same time increase the hatching rate of eggs of different fish species
Conclusion
In conclusion, the iodine appeared suitable for disinfecting turbot eggs if treatment is applied at the early morula stage. A concentration of 100 ppm during 10 minutes seems to be acceptable for a disinfection at 14 °C.
805
References
- Alderman, D.J., (1984). The toxicity of iodophors to salmonid eggs, Aquaculture, 40: 7-16. doi:10.1016/0044-8486(84)90211-4
- nAmend, D.F., Pietch, J.P., (1972). Virucidal activity of two iodophors to salmonid viruses, Journal of the Fisheries Research Board of Canada, 29: 61-65
- nBarker, G.A., Smith, S.N., Bromage, N.R., (1989). The bacterial flora of rainbow trout, Salmogairdneri Richardson, and brown trout, Salmotrutta L. eggs and its relationship to developmental success, Journal of Fish Diseases, 12: 281-293. doi:10.1111/j.1365-2761.1989.tb00317.x
- nBatts, W.N., Landolt, M.L., Winton, J.R., (1991). Inactivation of hematopoietic necrosis virus (IHNV) by low levels of iodine, Applied and Environmental Microbiology, 57(5): 1379-1385
- nBergh, Ø.,Jelmert, A., (1996). Iodophor disinfection of eggs of Atlantic halibut, Journal Aquatic Animal Health, 8:133-145. doi:10.1577/1548-8667(1996)008<0135:IDOEOA>2.3.CO;2
- nBromage, N.R., (1995). Broodstock management and seed quality—general considerations. In: Bromage, N.R., Roberts, R.J. (Eds.), Broodstock Management and Egg and Larval Quality. Blackwell Science, Oxford, pp. 1–24
- nDavenport, J., Lønning, S., Kjørsvik, E., (1986). Some mechanical and morphological properties of the chorion of marine teleost eggs, Journal of Fish Biology, 29: 289–301. doi:10.1111/j.1095-8649.1986.tb04946.x
- nFowler, L.G., Banks, J.L., (1990). Iodophor toxicity to eggs and fry of fall Chinook salmon, The Progressive Fish-Culturist, 52: 176-178. doi:10.1577/1548-8640(1990) 052<0176:CITTEA>2.3.CO;2
- nFowler, L.G., Banks, J.L., (1991). A safe level of iodophor for treating eggs of fall chinook salmon during water hardening, The Progressive Fish-Culturist, 53: 250-251. doi:10.1577/1548-8640(1991)053<0250:TNASLO>2.3.CO;2
- nGoldes, S.A., Mead, S.L., (1995). Efficacy of iodophor disinfection against egg surfaceassociated infectious hematopoietic necrosis virus, The Progress Fish Culture 57: 26-29. doi:10.1577/1548-8640(1995)057<0026:AEOIDA>2.3.CO;2
- nHarboe,T., Huse,I.,Oie,G., (1994). Effects of egg disinfection on yolk-sac and 1st feeding stages of halibut (Hippoglossushippoglossus L.) larvae, Aquaculture, 119: 157–165. doi:10.1016/0044-8486(94)90172-4
- nHirazawa, N., Hara, T., Mitsuboshi, T., Okazaki, J., Hata, K., (1999). Iodophor disinfection of eggs of spotted halibut Veraspervariegatus and red seabreamPagrus major, Fisheries Science, 65(3): 333-338
- nKjørsvik, E., Mangor-Jensen, A., Holmefjord, I., (1990). Egg quality in fishes, Advances in Marine Biology, 26: 71–113.doi:10.1016/S0065-2881(08)60199-6
- nLønning, S., Kjørsvik, E., Falk-Petersen I. B., (1988). A comparative study of pelagic and demersal eggs from common marine fishes in northern Norway, Sarsia, 73: 49–60
- nMcFadden, TW., (1969). Effective disinfection of trout eggs to prevent transmission of Aeromonasliquifaciens, Journal of the Fisheries Research Board of Canada, 26: 2311- 2318
- nMuroga, K., (1995). Viral and bacterial disease in larval and juvenile marine fish and shellfish: A review, Fish Pathology, 30: 71-85
- nPeck, M.A., Buckley, L., O'Bryan, L.M., Davies, E.J., Lapolla, A.E., (2004). Efficacy of egg surface disinfectants in captive spawning Atlantic cod Gadusmorhua L. and haddock Melanogrammusaeglefinus L., Aquaculture Research, 35: 992–996. doi:10.1111/j.1365-2109.2004.01119.x
- nSalvesen, I., Jørgensen, L, and Vadstein, O., (1991). Evaluation of four chemicals for surface disinfection of marine fish eggs. In: Lavens P, Sorgeloos P, Jaspers E, andOllevier F (eds). Larvi ‘91-Fish and Crustacean Larviculture Symposium, European Aquaculture Society, Special Publication No. 15, Gent Belgium
- nSalvesen, I., Øie, G., Vadstein, O., (1997). Surface disinfection of Atlantic halibut and turbot eggs with glutaraldehyde: Evaluation of concentrations and contact times, Aquaculture International, 5: 249–258. doi:10.1023/A:1018343602872
- nSauter, R.W., Williams, C., Meyer, E.A., Celnik, B., Banks, J.L., Leith, D.A., (1987). A study of bacteria present within unfertilized salmon eggs at the time of spawning and their possible relation to early lifestage disease, Journal of Fish Diseases, 10: 193- 203.doi:10.1111/j.1365-2761.1987.tb01061.xnSavaş, H., Türe, M. (2007). Bölgemizdedoğalvekültürüyapılanbalıklardagörülenhastalıklar, SÜMAE YunusAraştırmaBülteni, 7(2): 10-13
- nSchachte, J.H., (1979). Iodophor disinfection of muskellunge eggs under intensive culture in hatcheries, The Progressive Fish-Culturist, 41: 189–190. doi:10.1577/1548-8659(1979) 41[189:IDOMEU]2.0.CO;2
- nSubasinghe, R.P., Sommerville, C., (1985). Disinfection of Oreochromis mossambicus (Peters) eggs against commonly occurring potentially pathogenic bacteria and fungi under artificial hatchery conditions, Aquaculture Research, 16: 121-127. doi:10.1111/j.1365-2109.1985.tb00301.x
- nSuquet, M., Billard, R., Cosson, J., Normant, Y., Fauvel, C., (1995). Artificial insemination in turbot (Scophthalmusmaximus): determination of the optimal sperm to egg ratio and time of gamete contact, Aquaculture, 133: 83–90. doi:10.1016/0044-8486(94)00395-5
- nTendencia, E.A., (2001). Effect of iodine disinfection on the bacterial flora and hatching rate of Epinepheluscoioides eggs at the cleavage and eyed stages, Bulletin European Association of Fish Pathologists, 21(4): 160- 163
- nTendencia, E.A., (2003). Iodine disinfection of grouper Epinepheluscoioides eggs, Bulletin European Association of Fish Pathologists, 23(4): 191-196
- nWright, L.D. and Snow, J.R., (1975). The effect of six chemicals for disinfection of largemouth bass eggs, The Progressive Fish Culturist, 37: 213-217. doi:10.1577/1548-8659(1975)37[213:TEOSCF]2.0.CO;2
- nZar. J.H., (1999). Biostatistical Analysis, 4th ed., Prentice Hall, New Jersey, 929 p.








