Keywords
Fish, Mrigal, Disease, Bacteria, Probiotic
Introduction
Probiotics, which are micro-organisms or their products, are used for the health welfare of the host. Recently probiotics have found their use as alternative agents to control the fish diseases. A wide range of microalgae, yeasts and bacteria have been isolated and used as probiotic in aquat-ic medium (Evenberg et al., 1985; Cahill, 1990; Mohanty et al., 1996; Liu et al., 2000; Alcaide, 2003; Ping Chung et al., 2004 and Austin and Brian, 2006). The mrigal (C. mrigala) is one of the Indian major carps which are an integral part of aquaculture and an important component of sustainable food security in India. These fishes are infected with wide variety of diseases, includ-ing the EUS. In the present context of conserva-tion of environment vis-à-vis ill effects of antibi-otics, new generations of preventive/ curative bi-oagents have come into force. To take the ad-vantage of these bioagents, the present investiga-tions were proposed to ascertain the effect of probiotics on various life parameters of mrigal (C. mrigala).
Materials and Methods
The samples of diseased fish were dissected, the affected tissue (skin lesions/muscles) was taken in a test tube, homogenized it into a ho-mogenizer and spread over the nutrient agar me-dium in Petri plates under aseptic conditions. These plates were incubated in B.O.D at 30 ± 1°C for 24 h. Bacterial growth on the nutrient agar plate was observed after 24 h. Pure colonies of bacteria were isolated and obtained further by sub-culturing of the single colonies on nutrient agar by proper streaking method (OIE, 2006). For the culture and isolation of the pathogenic bacte-ria, method suggested by OIE (2006) was fol-lowed. Commercially available probiotic was tested for their role as disease controlling agents against the infections caused by pathogenic fungi in C. mrigala. The composition of probiotic is Lactobacillus sporogenes, Lactobacillus acidophilus, Bacillus subtilis, Bacillus Licheniformis, Saccharomyces cervirial, Sea weed extract; Enzyme complex contains Amylase, Phylase, Protease, Cellulose, Beta-galactosidase, Lipase, C20g vitamin, Vitamin B61g, Sodium Benzoate.
In vitro test of probiotics
In vitro test of available probiotics for their antagonistic potential against fungus was done by using poisoned food technique (Verma et al., 2001). The basic principle of this technique was to poison the culture medium with pathogen and then allow the test probiotic to grow on such me-dium.
In vivo tests of probiotics
Aphanomyces invadans (fungus) were taken as pathogenic organisms for their inoculation in the mrigal (C. mrigala). Different treatments were given to the fish. The following treatments were given to the fish.
i) Control: In this treatment, 250μl of physio-logical buffer saline (PBS) was given into the intraperitoneal cavity of each acclimated fish.
iii) Control+fungus: Here, the motile spores of the fungus were dissolved/submerged into 1 ml of PBS solution. The concentration/μl of the spores was determined utilizing hemocy-tometer. The solution was then diluted up to 100 spores/250 μl of PBS. It was inoculated into the intraperitonal cavity of fish.
vi) Control+fungus+probiotic: In this case, 0.1gm probiotic1 and 100 motile spores of fungus, taken in 250 μl of PBS, were inocu-lated into the intraperitonal cavity of fish.
xi) Control+probiotic: Here, 0.1gm probiotic1 dissolved in 250 μl of physiological buffer saline was inoculated into the intraperitonal cavity of fish.
Hematological Studies
Different blood parameters viz. level of hae-moglobin (Hb), total erythrocyte count (TEC), total leucocytes count (TLC) hemotocrit/packed cell volume (PCV) were determined with help of a haemocytometer and calculated from the equa-tions given by Anderson and Klontz (1965).
Collection of blood
Blood samples of treated fish were taken at weekly interval after initiation of treatments. Sampling was also done at the same time from control group. Blood was drawn from the caudal peduncle region using a sterile syringe of 2 ml rinsed with 2.7% Ethylene dimethyl tetra amine (EDTA) solution. Blood was collected in small glass vials after drying the vials in hot air oven.
a) Haemoglobin estimation in the blood of mrigal (C.mrigala) under different treat-ments: The hemoglobin contents of blood were analyzed following the Cyanmethemoglobin methods using Darbkins Fluid. Twenty micro liter of blood was mixed with 5 ml Darbkin’s working solution. The absorbance was measured using a spectrophotometer at wavelength of a 540 nm. Hemoglobin con-tents were expressed as g / d1.
b) Total erythrocyte count: The blood was drawn from the caudal vein and EDTA was used as an anticoagulant to prevent the blood cells from lysis and clotting. The blood was diluted to 1:200, with RBC counting pipette. The mixture was shaken well to suspend the cells uniformly in the solution. Then the cells were counted using a haemocytometer as follows:
Number of RBC/mm3 = N x 10000 (where, N= total number of red blood cells counted in 5 squares of the haemocytometer slide and 10,000 is the dilution factor).
c) Total leukocyte count: The blood was drawn from the caudal vein and EDTA was used as an anticoagulant. Blood was diluted 1:20 with WBC diluting fluids using WBC counting pi-pette. The mixture was shaken well to suspend the cells uniformly in the solution. Then the cells were counted using a haemocytometer as follows:
Number of WBC/mm3 = N x 50 (where, N = total number of white blood cells counted in 4 squares of the hemocytometer slide and 50 is dilution factor).
Statistical analysis
The obtained results were analyzed statistical-ly using completely randomized design (CRD) to evaluate differences among different treatments means at 0.05 significant levels following Snedecor and Cochran (1989).
Results and Discussion
Level of hemoglobin in the blood of mrigal (C. mrigala) under different treatments
The results on hemoglobin level in the blood of mrigal (C. mrigala) under different treatments over a period of eight weeks are presented in Table 1. The hemoglobin level of normal fish re-mained in the range of 6.27 to 6.55 g/ 100ml. However, in fishes inoculated with fungus alone, the level of hemoglobin fell drastically and re-mained in the range of 4.17 to 2.34 g/ 100ml. The hemoglobin level increased in the range of 4.91 to 6.62 in fish inoculated with fungus + probiotic, respectively. On the other hand, the fish given the treatment of probiotic showed maximal value of haemoglobin level as compared to all other treatments including control. The hemoglobin level was in the range of 6.67 to 7.35 in fish ad-ministrated with probiotic. These results revealed that probiotic gives better results in increasing the hemoglobin level of fish.
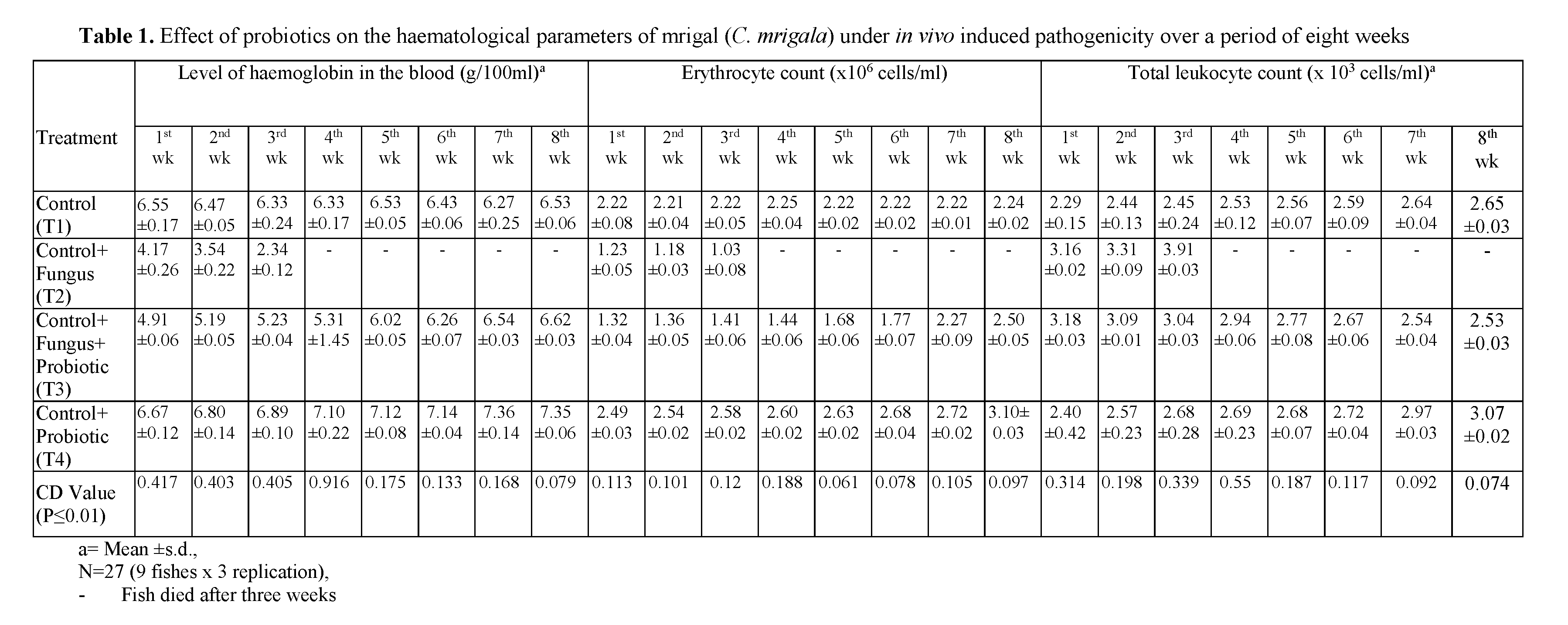
Table 1: Effect of probiotics on the haematological parameters of mrigal (C. mrigala) under in vivo induced pathogenicity over a period of eight weeks
Level of total erythrocyte count (TEC) in the blood of mrigal (C. mrigala) under different treatments
The results on erythrocyte count level in the blood of mrigal (C. mrigala) under different treatments over a period of eight weeks are pre-sented in Table 1. The erythrocyte count level of normal fish remained in the range of 2.21 to 2.25. However, in fishes inoculated with pathogenic alone, the level of erythrocyte count fell and re-mained in the range of 1.23 to 1.03. The erythro-cyte count level increased in the range of 1.32 to 2.50 in fish inoculated with fungus along with probiotic. However, the erythrocyte count level was in the range of 1.49 to 2.58 in fish inoculated fungus along with probiotic. On the other hand, the fish given the treatment of probiotic showed maximal value of erythrocyte count level as compared to all other treatments. The erythrocyte count level was in the range of 2.49 to 3.10 in fish administrated with probiotic.
Level of total leukocyte count (TLC) in the blood of mrigal (C. mrigala) under different treatments
The results on leukocyte count level in the blood of mrigal (C. mrigala) under different treatments over a period of eight weeks are pre-sented in Table 1. The leukocyte count level of normal fish remained in the range of 2.29 to 2.65. However, in fishes inoculated with fungus alone, the level of leukocyte increased and remained in the range of 3.16 to 3.91.
The leukocyte count remained in the range of 2.46 to 2.94 in fish inoculated with fungus + pro-biotic. However, the leukocyte count level was in the range of 2.53 to 3.18 in fish inoculated with fungus along with probiotic. On the other hand, the fish given the treatment of probiotic showed maximal value of leukocyte count as compared to all other treatments including control. The level of leukocyte count was in the range of 2.40 to 3.07 in fish administrated with probiotic. These results revealed that probiotic gives better results in increasing the leukocyte count of fish.
Hematological parameters of fish reflect the gravity of these changes. Values of hematological parameters of fishes can be affected by environ-mental and biological factors such as age, weight, sex, food, bacteria, viruses, fungi and water quality parameters (Das and Das, 1993). Palikova et al. (2004) observed decrease in the level of blood in the common carp after exposure to Cya-nobacteria extract. Results of all these studies re-semble those of the present investigation showing that fish inoculated with pathogenic bacteria and fungus showed a decrease in its blood parame-ters. Hemoglobin level reduced approximately to 50% in its value; erythrocytes count reduced ap-proximately to 40% in its value; leucocytes count reduced to approximately 40% in its value and packed cell volume reduced approximately to 40% in its value (Table 1) in three weeks. This clearly indicated a marked decline in the hemato-logical parameters of diseased fishes.
An increase in erythrocyte count in fish, fed on probiotic bacteria than control group observed in fish species (Irianto and Austin, 2002; Selvaraj et al., 2005; Rengpipat et al., 1998 and Prabhu et al., 1999). The results of present study revealed that probiotic had a positive effect on hemoglobin level increased approximately to 50% in its val-ue; erythrocytes count increased approximately to 40% in its value; leukocyte count increased ap-proximately to 30% in its value (Table 1). This clearly indicated that there was increased in the value of hematological parameters of fish. The mean values of various hematological parameters in different treatments groups of fish during the experiment are presented in Table 1. At the end of third week, all the fish died.
Conculisions
The present study also confirmed the findings of Mae da and Liao (1992) and Garriques and Are-valo (1995) who reported a significant increment in growth of Penaeus monodon and Penaeus vannamei fed with probiotic incorporated feeds. This study therefore, clearly reveals that probiot-ics are very effective in controlling the fish dis-eases in order to improve their health status. The present study further shows that fishes treated with probiotics showed increase in the level of different hematological parameters viz. hemoglo-bin, erythrocytes count, leucocytes count and packed cell volume of fish significantly.
Acknowledgements
We are thankful to the Head, Department of Zoology and Aquaculture, CCS Haryana Agricul-tural University, Hisar, for providing the neces-sary facilities. The financial assistance rendered to me in the form of Merit Stipend by CCS Har-yana Agricultural University, Hisar, is gratefully acknowledged.
432
References
- Alcaide, E., (2003). Numerical taxonomy of Vibri Lonaceae isolated from cultured am-berjack (Seriola dumerili) and surrounding water, Current Microbiology, 46: 184–189. doi: 10.1007/s00284-002-3826-2
- nAnderson, D., Klontz, G.W., (1965). Basic Hae-matology for the fish culturist, Annual Nortwest Fish Culture Conference, 16: 38-41
- nAustin, B., (2006). The Bacterial Microflora of Fish, Revised, The Scientific World Journal, 6: 931-945. doi:10.1100/tsw.2006.181
- nCahill, M.M., (1990). Bacterial flora of fishes: A review, Microbial Ecology, 19: 21–41. doi: 10.1007/BF02015051
- nDas, M.K., Das, R.K., (1993). A review of the fish disease epizootic ulcerative syndrome in India, Environment & Ecology, 11: 134-145
- nEvenberg, D., Versluis, R., Lugtenberg, B., (1985). Biochemical and immunological characterization of the cell surface of the fish pathogenic bacterium Aeromonas salm-onicida, Biomembranes, 815(2): 233-244. doi: 10.1016/0005-2736(85)90294-9
- nGarriques, D., Arevalo, G., (1995). An evaluation of the production and use of a live bacterial isolate to manipulate the microbial flora in the commercial production of Penaeus vannzamei postlarvae in Ecuador. C. L. Browdy and J. S. Hopkins, editors. Swim-ming through troubled water, proceedings of the special session on shrimp farming. World Aquaculture Society, pp. 53-59
- nIrianto, A., Austin, B., (2002). Probiotic in aqua-culture, Journal of Fish Disease, 25: 633-642. doi: 10.1046/j.1365-2761.2002.00422
- nKennedy, S.B., Tucker, J.W., Thoresen, M., Sen-nett, D.G., (1998). Current methodology for the use of probiotic bacteria in the culture of marine fish larvae, (Aquaculture 98, World Aqua. Soc., Baton Rouge), pp. 286
- nKeskin, O., Secer, S., Izgor, M., Turkyilmaz, S., Makaosya, R.S., (2004). Edwardsiella ic-taluri infection in Rainbow Trout (On-corhynchus mykiss), Journal Veterinary An-imal Science, 28: 649-653
- nLiu, P.C., Yeh, Y.P., Lee, K.K., (2000). Patho-genicity of Vibrio harveyi isolated from yel-lowfin seabream Acanthopagrus latuswith red-gut syndrome, Reports of Fish Disease Research, 20: 79-86
- nMaeda, M., Liao, I.C., (1992). Effect of bacterial population on the growth of a prawn larva, Penaeus monodon, Aquaculture, 21: 25-29
- nMohanty, S.N., Swain, S.K., Tripathi, S.D., (1996). Rearing of catla (Catla catla) spawn on formulated diets, Aquaculture, 11: 253-258. doi: 10.1111/j.1365-2095.2011.00866.x
- nPalikova, M., Navratil, S., Krejcf, R., Sterba, F., Tichy, F., Kubala, L., (2004). Outcomes of repeated exposure of carp (Cyprinus carpio L) to Cyanobacteria extract, Acta Veterinar-ia Brno, 73: 259-265
- nPing Chung L., Ji-Yang, L., Wen Hsiao, C., Kuo Kau, L., (2004). Isolation and Characterization of Pathogenic Vibrio harveyi (V. car-chariae) From the Farmed Marine Cobia Fish Rachycentron canadum L. with Gastro-enteritis Syndrome, World Journal of Mi-crobiology and Biotechnology, 20: 495-499. doi: 10.1023/B:WIBI.0000040402.44340.0e
- nPrabhu, N.M., Nazar, A.R., Rajagopal, S., Ajmal-Khan, S., (1999). Use of probiotics in water quality management during shrimp culture, Journal of Aquaculture Tropics, 14(3): 227-236
- nRengpipat, S., Rukpratanporn, S., Piyatiratitivo-rakul, S., Menasaveta, P., (1998). Immunity enhancement in black tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11), Aquaculture, 191: 271-288. doi: 10.1016/S0044-8486(98)00305-6
- nSelvaraj, V., Sampath, K., Sekar, V., (2005). Administration of yeast glucan enhances survival and some non-specific and specific immune parameters in carp (Cyprinus car-pio) infected with A. hydrophila, Fish and Shellfish Immunology, 19: 293-306. doi: 10.1016/j.fsi.2005.01.001
- nSnedecor, G., Cochran, W., Cox, D., (1989). Sta-tistical Methods (8th edition). The Iowa State University Press
- nVerma, A., Kukreja, K., Pathak, D.V., Suneja, S., Narula, N., (2001). In vitro production of plant growth regulators by Azotobacter chroococcum, Indian journal Microbiology, 41: 305-307.







