Keywords
Clove oil, phenoxyethanol, enzymes
Introduction
Anesthesia is a process which is used before performing an operation on a living organism, to prevent pain caused by the operation, to slow down the metabolism rate by decreasing and re-moving the sensation and consciousness and to slow down or stop the reflex reactions of the or-ganism (Çetinkaya, 1991; Sisecio?lu et al., 2009). Enzymes are the biological catalysts which are synthesized by living cells and speed up the chemical reactions in the living metabo-lism and bring 100% product efficiency without producing any by-products (Lehninger, 2005). They are synthesized inside the cell and are used in accordance with their purposes. But, in some pathological cases, in the intercellular fluid or blood plasma, the enzyme level increases. It has been informed that the reason for this can be ei-ther the increase of enzyme synthesis, or the in-crease in cell membrane permeability or it can be because of the decomposition of cell, in other words cell necrosis (Lehninger, 2005).
The active substance of 2-phenoxyethanol is ethylene glycol monophenyl ether. Its summary formula is C8H10O2, the molar weight 138.17 gl-1, density 1.107-1.108 g·dm-3, peroxide content less than 0.005% and the boiling temperature is 245°C. The anesthetic is slightly soluble in water (26.7 gl-1) at 25°C but readily soluble in ethanol. The anesthetic affects fish through the skin and gills.
Main advantages of clove oil can be pointed out as; its regulating attribute is limited, its excreting time from the body is short, it is rather safe for human and animals, it can be provided easily and it is inexpensive. It almost meets every measure that should be considered during the selection of anesthetics (Marking et al., 1985; Keene et al., 1998).
Besides this, the most important disad-vantages of clove oil can be pointed out as fol-lows; its application for a long time would affect the oxygen consumption, it causes color changes in perch, brown trout and atlantic salmon; it causes equilibrium loss for fishes when there is small concentration increases at different carriage conditions in large carrying tanks and it is hard to control the concentration (Hoskonen et al., 2004), the long time of exposure during carriage causes increase of ammonia level. It is mixed with solvents as alcohol as it is not dissolved in water and when it should be used the least dosage that pro-duces anesthesia should be used (Kaiser et al., 2002).
Antioxidant defense system protects the cell from the oxidative damage of free radicals or other reactive molecules (Fridovich, 1976). Anti-oxidants synthesized in the body may also be taken with diet from the outside. Antioxidant de-fense systems in living organisms are divided in-to two main groups. These are produced in me-tabolism (endogenous) and outside of the diet (exogenous) antioxidant systems (Gulcin, 2002). Endogenous antioxidant system consists of anti-oxidant enzymes, phospholipase and damaged molecules repellent proteases. Endogenous anti-oxidants in the structure of the enzyme remove oxidative damage (Fridovich, 1976). Therefore in this defense system, antioxidant enzymes are very important (Gulcin, 2002). Glutathione re-ductase, catalase and peroxidases are very im-portant antioxidant enzymes. Catalase (CAT) (EC 1.11.1.6) is a characteristic enzyme that is abundant in protein structure. This enzyme is widely present in animals, plants and microor-ganisms. Also, it plays an important role in re-moval of toxic hydrogen peroxide from the cells (De Duve, 1983).
Catalase makes the conversion of hydrogen peroxide to water as a one way reaction (Kunce et al., 1986; Havir et al., 1987; Keha et al., 2007) and includes each group as prosthetic group (Kar et al., 1976; Yamaguchi et al., 1984). Glutathione reductase (GR) (EC 1.8.1.7) enzyme catalyzes the electron transfer between low or high molec-ular weight disulphate substrates and reduced pyridine nucleotides. One of the most important targets of glutathione reductase enzyme that is known is to keep the ratio of GSH (Reduced glu-tathione)/GSSG (Oxidized glutathione) in the cell medium (Keha et al., 2007). In clinical chemistry, glutathione reductase is used in determination of hepatic disorders and cancer, nutrition, measure-ment of riboflavin deficiency and determination of some genetic deficiencies (Beutler, 1969). 6-Phospho glyconate dehydrogenase (6PGD) (EC 1.1.1.44) is responsible from very important mol-ecules in living organisms NADPH and ribose 5-phosphate and any defect related with the enzyme would bring many conditions that would be nega-tive for them. Deficiency of 6PGD negatively af-fects glucose metabolism in the cell. Because of its vital importance, 6PGD has to be purified from several types and its structural and kinetic characteristics should be determined (Tandogan et al., 2003). Glucose 6-phosphate dehydrogenase (G6PD) (E.C. 1.1.1.49) is one of the most im-portant enzymes of pentose phosphate pathway. The reaction catalyzed by this enzyme is the first reaction of pentose phosphate pathway and con-stitutes the control point. It is present in almost every animal tissue, plant, yeast and microorgan-isms (Anderson et al., 1974; Bergmeyer et al., 1983; Lehninger, 2005). The main physiological function of these two enzymes are to produce NADPH, which is essential for reductive bio-synthesis and nucleic acid synthesis and protects the cell against oxidants by producing reduced glutathione (GSH). Therefore these two enzymes are similar to antioxidant enzymes (Kuo et al., 2000; Bianchi et al., 2001). The studies that are performed to understand the physiological re-sponses of fishes against the stress elements (stock density, temperature, oxygen etc.) that they are exposed to, their disease conditions, sev-eral drugs, heavy metals and pollutants as pesti-cides are mostly based on hematological, bio-chemical and histopathological methods (Pick-ering et al., 1987; Asztalos et al., 1990; Sohlberg et al., 1990; Mazur et al., 1993; Shakoori et al., 1994). However, none of the used methods alone are satisfactory for understanding the physiologi-cal responses of fishes.
This study is performed in order to build up the required basis to accurately determine the physiological responses in in vivo medium by measuring activity of some enzymes (GR, G6PD, 6PGD, CAT) from the blood samples taken after the application of anesthetic substances (clove oil and phenoxyethanol) to rainbow trout and brown trout. It is aimed to demonstrate that clove oil, which is an organic anesthetic, has no deficiency when compared with its synthetic equivalents and it has no negative effect on the important fish species in inland water fish farming.
Materials and Methods
Experimental groups of 48 fish with an aver-age weight of 180 ±25 g, reared in well water with a constant temperature of 8.5°C at our farm, located at the Research and Extension Center in Atatürk University, was transferred to the Central Laboratory in the Aquarium Fish Rearing Facility and was exposed to a anesthesia dose of 0.5 ppm of clove oil and 0.2 ppm of 2-phenoxyethanol for 4 weeks in circular fiberglass tanks 780 lt in vol-ume (100 cm diameter and 100 cm depth) under natural lighting conditions with a constant flow (1.5-l min-1) of aerated dechlorinated tap water at 9-11°C and with no recirculation. The dissolved oxygen and pH levels and total water hardness were 8-9 ppm, 7.8 and 102 mg in CaCO3, re-spectively. The tanks were aerated with an air pump.
Eight fish were placed into each of 6 tanks, for testing the clove oil, 2-phenoxyethanol and the other as the control. At the end of each day of exposure, 8 fish from the control tank, 8 fish from the clove oil treatment tank and 8 fish from the 2-phenoxyethanol tank (Ahmad et al., 1995; Shakoori et al., 1996) were taken out and their blood was subjected to hematological analysis (Shakoori et al., 1996; Santhakumar et al., 1999; Atamanalp et al., 2002).
Clove oil, and 2-phenoxyethanol application of anesthetic agent’s forms:
Completely insoluble in water bath, clove oil used in the study of anesthesia in 95% ethanol, diluted to 1:10 ratio. Anesthesia; 0.5 ml liter tank was created by adding the anesthetic bath. 2-phe-noxyethanol was diluted 1:10 with ethanol bath and 0.5 ml / l was prepared by adding phenoxy-ethanol. Both anesthetic baths were applied for 1 minute and the fish were stored in clean water tanks. And recovery phases of anesthesia were recorded by monitoring the process.
Measurement of Enzyme Activities
Measurement of G6PD and 6PGD Enzyme Ac-tivity:
Glucose 6-phosphate dehydrogenase (G6PD) ac-tivity was determined by monitoring NADPH production at 340 nm and 25°C. The assay mix-ture contained 10 mM magnesium chloride, 0.2 mM NADP+, and 0.6 mM G6PD in 100 mM Tris-hydrochlorid buffer solution at pH 8.0. Assays were carried out in triplicate and the activities were followed up for 60 s. One unit of activity (U) is defined as the amount of enzyme required to reduce 1 μmol/min of NADP+ under the assay conditions (Beutler, 1971).
Measurement of Glutathione Reductase Enzyme Activity:
Glutathione reductase enzyme activity was meas-ured by Beutler’s method. One enzyme unit is de-fined as the oxidation of 1 mmol NADPH per min under the assay condition (25°C, pH 8.0) (Beutler, 1971).
Measurement of Catalase Enzyme Activity:
The catalase activity was measured by the Aebi method. In this method, 20 ml enzyme solution was added to the 1 ml 10mM H2O2 in 20 mM po-tassium phosphate buffer (pH 7.0) and incubated at 250C for 1 min. Initial reaction rate was meas-ured from the decrease in absorbance at 240 nm (Aebi, 1984).
Statistical analysis
The results are presented as means ± SD. Differ-ences between parameters were analyzed by one-way analysis of variance (ANOVA), and signifi-cant means were subjected to a multiple compari-son test (Duncan) at the a = 0.05 level (Hayran et al., 1995).
Results and Discussion
The variance analysis table for mean values of G6PD enzyme is given in table 1, mean G6PD values according to types are given in table 2 and G6PD values of groups are given in Figure 1. In the control group G6PD enzyme value was found as 2.170±1.477 for brown trout and 1.848±0.113 for rainbow trout. When phenoxyethanol was ap-plied, for brown trout this value was determined as 6.430±0.227, and for rainbow trout 6.591±1.491. For clove oil group, the mentioned parameter was found as 6.591±3.410 for brown trout and 6.109±2.955 for rainbow trout.
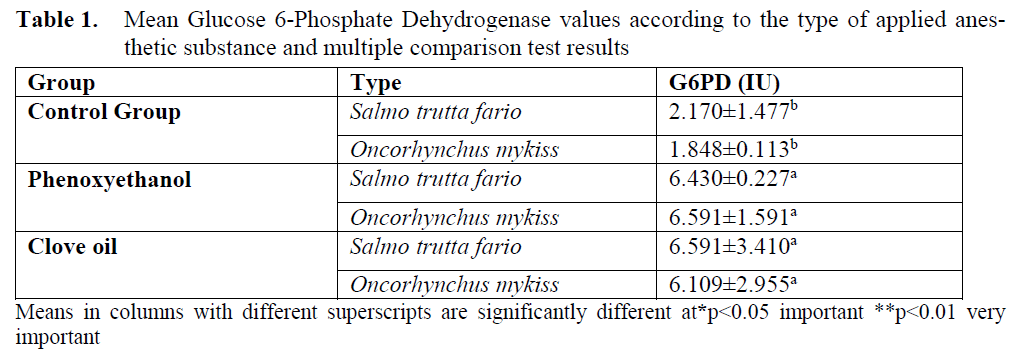
Table 1. Mean Glucose 6-Phosphate Dehydrogenase values according to the type of applied anes-thetic substance and multiple comparison test results
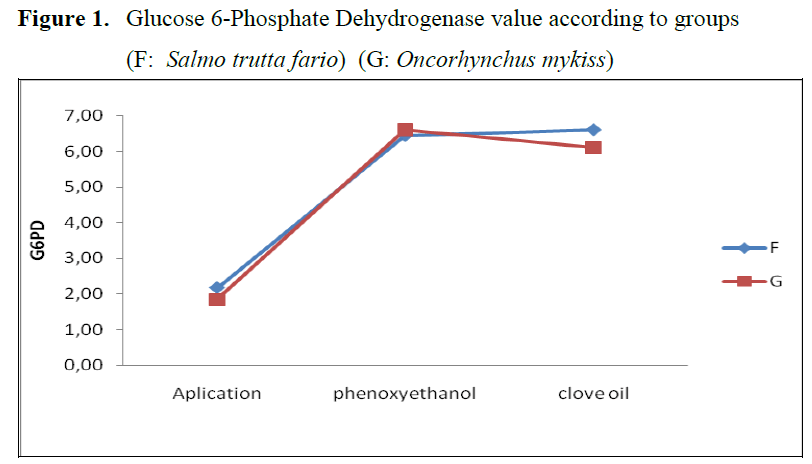
Figure 1. Glucose 6-Phosphate Dehydrogenase value according to groups
Variance analysis table for 6PGD enzyme mean values is given in table 2. 6PGD enzyme value of the control group was found as 2.049±1.193 for brown trout and 2.049±1.534 for rainbow trout. This parameter was found as 4.702±2.557 for brown trout and 6.511±1.705 for rainbow trout from the application groups for which phenoxyethanol was applied. In clove oil group, for brown trout 6PGD enzyme value was found as 4.702±2.557 and for rainbow trout it is found as 6.993±1.705.
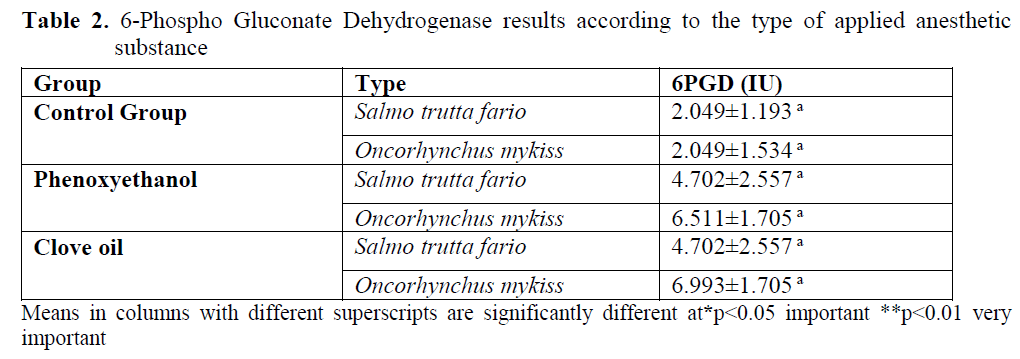
Table 2. 6-Phospho Gluconate Dehydrogenase results according to the type of applied anesthetic substance
Mean GR and CAT enzymes values are given in table 3- table 4 were observed as 0.602±0.284 for brown trout, 1.004±0.852 for rainbow trout. This parameter was found as 0.602±0.284 for brown trout and 1.004±0.284 for rainbow trout from the application groups for which phenoxy-ethanol was applied. In clove oil group, for brown trout GR enzyme value was found as 0.401±0.260 and for rainbow trout it was found as 1.808±0.852.
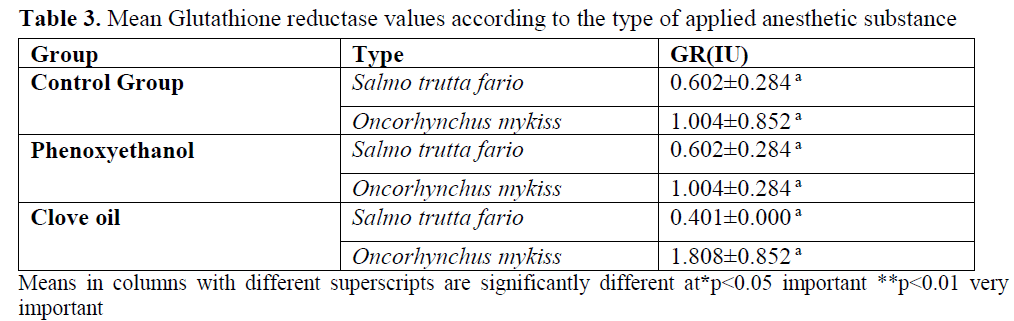
Table 3. Mean Glutathione reductase values according to the type of applied anesthetic substance
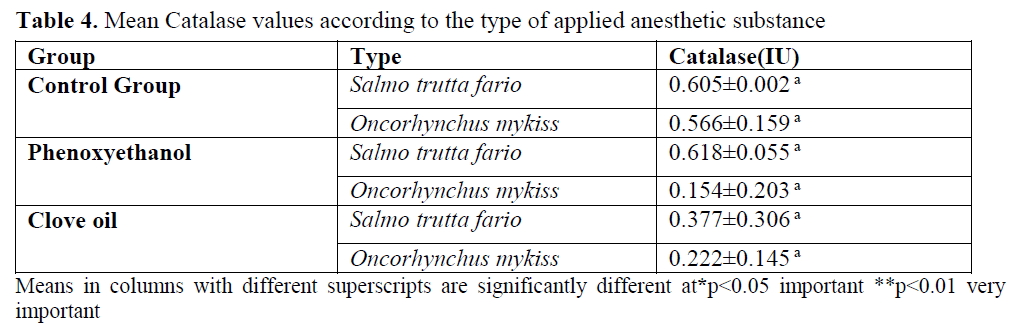
Table 4. Mean Catalase values according to the type of applied anesthetic substance
CAT enzyme value of the control group was found as 0.605±0.02 EU/g for brown trout and 0.566±0.159 EU/g for rainbow trout. In the ap-plication groups, phenoxyethanol application gave 0.618±0.055 EU/g value for brown trout and 0.154±0.203 EU/g value for rainbow trout. In clove oil group for brown trout was 0.377±0.306 EU/g and for rainbow trout was 0.222±0.145 EU/g.
The importance of G6PD and 6PGD in me-tabolism has been well known for many years. GSH is used by antioxidant defense mechanisms and its production requires NADPH to be synthe-sized in the pentose phosphate metabolic path-way in which G6PD and 6PGD participate. For this reason, G6PD and 6PGD are considered as antioxidant enzymes (Reiter et al., 1997).
According to the results given in Table 1, both application groups, both application groups have high G6PD enzyme values. Direct measurement of stress may be performed by the measurement of secreted hormones. But this type of approach is fairly expensive. Instead, it is possible to have information about the encountered stress level by (secondary) determination of physiological changes happened in blood and tissues after the stress response. Determination of enzymes in se-rum which indicate organ or tissue damage, glu-cose level in blood (indirect indicator of hormo-nal activity), and CI amount in blood as the ion regulation indicator can be used in measurement of stress effect for different places and purposes (Ogut, 2005). G6PD activity changes depending on nutrition, hormones and especially NADPH concentration. Ozmen et al., 2004 inspected the effects of recirculation system on antioxidant en-zymes of rainbow trout and when G6PD enzyme is compared with control group the result were found very important. In the application groups, a significant increase was observed in G6PD en-zyme activity as it stops the enzyme synthesis.
6-phosphogluconate dehydrogenase is the third enzyme of the pentose phosphate pathway which converts 6-phosphogluconate compound to D-ribulose 5-phosphate in the presence of NADP+. The importance of the enzyme is be-cause of its reduction of NADP+ to NADPH. In many microorganisms it takes charge as glucose and gluconate catabolic enzyme (Yoshida et al., 1997).The enzyme is important in keeping the equilibrium between glycolytic and pentose phosphate pathway of glucose 6-phosphate (Delmar et al., 1986). As a result of phenoxy-ethanol and clove oil application, it is determined that the values for brown trout and rainbow trout are found to be close to each other and higher than the values determined for control group. When statistically examined the difference be-tween application, species and application x spe-cies interaction is found to be insignificant.
The results of this in vivo experiment indicate that an increase in the activities of G6PD and 6PGD enzymes in the rainbow trout, brown trout were induced in the presence of clove oil, 2-phe-noxyethanol. These increased enzyme activity levels ultimately served to protect fish from oxi-dative stress by increasing the level of GSH be-cause the regeneration of GSH from glutathione disulfide (GSSG) depends on the presence of glu-tathione reductase (GR) and NADPH in the envi-ronment; high activity of the NADPH-generating enzymes, G6PD and 6PGD, leads to an increase in GSH levels (O’Brien et al., 2001).
Catalese and glutathione reductase enzymes directly effects radicals. When statistically ex-amined the effect of application, species and ap-plication x species interaction on GR is found to be insignificant. The effects of recirculation sys-tem on antioxidant enzymes of rainbow trout and when GR enzyme is compared with control group the result were found very important (Ozmen et al., 2004).
Compared with control group for catalase en-zyme, the values obtained by application of phe-noxyethanol with brown trout are close to nor-mal, but lower in rainbow trout. It has been found that the catalase enzyme value obtained after clove oil application is less than control group for brown trout and rainbow trout. Catalase value has not been affected by application, species and ap-plication x species interaction. In sea bream it was reported that organic pollutants increase the catalase activity (Uner et al., 2001; Sayeed et al., 2003). This case shows parallelism with the re-sult of the study. But the same chemical has decreased catalase activity in Channa punctatus. It is reported that the seasons are effective in cata-lase activity (Ronisz et al., 1999).
As a result, the necessity of using anesthetics and sedatives in many stages of farming and the advantages it provides make the usage of anes-thetics inevitable. But using anesthetics is an ap-plication that needs experience and great care. The anesthetic should be not only chosen and used according to the aim, but also its effects on human health should be known very well. Cur-rently, there is only one anesthetic which is approved by FDA. This is MS-222. Recently, an anesthetic called as aqui-s has been approved by FDA and is used in several countries freely. However, these fishes can be submitted to human consumption 21 days after using anesthetics (Se-rezli et al., 2003).
Anesthetics provide important ease in many fish handling applications and clove oil is consid-ered as a good choice against synthetic anesthet-ics.
Conclusion
In this study it was determined that when the natural and synthetic anesthetic substances were used in recommended dosages, they had no neg-ative effects on the blood biochemistry, hematol-ogy and enzyme activities of both fish species. In accordance with these results, in fish farming ac-tivities it was determined that clove oil is equiv-alent to 2-phenoxyethanol substance in every re-spect, practically have ease of use and economi-cally have more advantages. Nowadays, the or-ganic agricultural products gain importance and the clove oil which is a natural compound will come into prominence in fish farming.
503
References
- Aebi, H., (1984). Catalase in vitro, Methods in Enzymology, 105: 121-126. doi: 10.1016/S0076-6879(84)05016-3
- nAhmad, F., Ali, S.S., Shakoori, A., (1995). Sub-lethal effects of danitol (Fenpropathrin), a synthetic pyrethroid, on freshwater Chinese grass carp, Ctenopharyngodon idella. Folia Biology, 43: 151-159
- nAnderson, L.E., Lim, N.G.T.C., Park, K.E.Y., (1974). Inactivation of pea leaf chloroplastic and cytoplazmic glucose-6-phosphate dehy-drogenases by light and dithiothreitol, Plant Physiology, 53: 835-839. doi: 10.1104/pp.53.6.835
- nAsztalos, B., Nemcsok, J., Benedeczky, I., Gabri-el, R., Szabo, A., Refaie, O.J., (1990). The effect of pesticides on some biochemical pa-rameters of carp (Cyprinus carpio), Archives of Environmental Contamination and Toxi-cology, 19: 275-282. doi: 10.1007/BF01056097
- nAtamanalp, M., Keles, M.S., Haliloglu, H.I., Aras, M.S., (2002). The effects of cyperme-thrin (A Synthetic Pyrethroid) on some bio-chemical parameters (Ca, P, Na and TP) of Rainbow Trout (Oncorhynchus mykiss), Turkish Journal of Veterinary and Animal Sciences, 26:1157-1160
- nBergmeyer, J., Grab, M., (1983). Methods of Enzymatic Analysis. Third Edition, 190-302
- nBeutler, E., (1969). Drug-induced hemolytic anemia, Pharmacological Reviews, 21: 73-103
- nBeutler, E., (1971). Red Cell Metabolism. A Manual of Biochemical Methods 12. Lon-don, Academic Press, 68-70
- nBianchi, D., Bertrant, O., Haupt, K., Coello, N., (2001). Effect of gluconic acid as a second-ary carbon source on non-growing L-lysine producers cells of Corynebacterium glu-tamicum. Purification and properties of 6-phosphogluconate dehydrogenase, Enzyme and Microbial Technology, 28: 754-759. doi: 10.1016/S0141-0229(01)00310-6
- nCetinkaya, O., (1991). Anesthesia application on fish and effects of water quality on anestehsia. Journal of Sour Agriculture, 2: 56-63
- nDeDuve, C., (1983). Microbodies in the living cells, Scientific American, 248: 2-5
- nDelmar, M., Puerta, M., Perlierra, A.G., (1986). Purification and properties of the enzyme 6-Phosphogluconate dehydrogenase from Di-centrarchus labrax liver. Comparative Phys-iology and Biochemistry, 83: 215-220
- nFridovich, I., (1976). In free radical in biology. New York, 1: 239-271
- nGulcin, I., (2002). Determination of antioxidant activity of nettle (Urtica dioica), characteri-sation and inspection of some in-vivo effects of oxidative enzymes. Atatürk University, Institute of Science, 37-55
- nHavir, E.A., Mchale, N.A., (1987). Biochemical and developmental characterization of mul-tiple forms of catalase in tobacco leaves, Plant Physiology, 84: 450-455. doi: 10.1104/pp.84.2.450
- nHayran, M., Özdemir, O., (1995). Bilgisayar, Istatistik ve Tip. Ankara, Hekimler Yayin Birligi, 484
- nHoskonen, P., Pirhonen, J., (2004). The effect of clove oil sedation on oxygen consumption of six temperate-zone fish species, Aquaculture Research, 35: 1002-1005.doi: 10.1111/j.1365-2109.2004.01084.x
- nKaiser, H., Brill, G., Cahill, J., Collett, P., Czypi-onka, K., Green, A., Orr, K., Pattrick, P., Woody, C.A., Nelson, J., Ramstad, K., (2002). Clove oil as an anaesthetic for adult sockeye salmon: field trials, Journal of Fish Biology, 60: 100-105
- nKar, M., Mishra, D., (1976). Catalase, peroxidase and polyphenoloxidase activities during rice leaf senescence, Plant Physiology, 57: 315-319.doi: 10.1104/pp.57.2.315
- nKeene, J.L., Noakes, D.L.G., Moccia, R.D., Soto, C.G., (1998). The efficacy of clove oil as an anaesthetic rainbow trout, Oncorhynchus mykiss (Walbaum), Aquaculture Research, 29: 89-101
- nKeha, E., Kufrevioglu, O.I., (2007). Biochemistry. Aktif Publishing House, 338-349
- nKunce, C.M., Trelease, R.N., (1986). Heterogeneity of catalase in maturing and germinated cotton seeds, Plant Pysiology, 81: 134-139.doi: 10.1104/PP.80.1.134
- nKuo, W., Lin, J., Tang, T.K., (2000). Human glu-cose-6-phosphate dehydrogenase (G6PD) gene transforms nih 3t3 cells and induces tumors in nude mice, The International Journal of Cancer, 85: 857-864.doi: 10.1002/(SICI)1097-0215(20000315)85:6<857::AID-IJC20>3.0.CO;2-U
- nLehninger, A.L., (2005). Biochemistry. New York, Worth Publishers, 764
- nMarking, L.L., Mayer, P.P., (1985). Are better anesths needed in fisheries, Fisheries, 10: 2-5
- nMazur, C.F., Iwama, O.K., (1993). Effect of han-dling and stocking density on haematocrit, plasma cortisol and survival in wild and hatchery-reared Chinook salmon (On-corhynchus tshawytscha), Aquaculture, 112: 291-299.doi: 10.1016/0044-8486(93)90390-K
- nO’Brien, M.L., Twaroski, T.P., Cunningham, M.L., Glauert, H.P., Spear, B.T., (2001). Ef-fects of peroxisome proliferators on antioxi-dant enzymes and antioxidant vitamins in rats and hamsters, Toxicological Sciences, 60: 271-278.doi: 10.1093/toxsci/60.2.271
- nOgut, H., (2005). Baliklarda Stres, Balik Biy-olojisi Arastirma Yöntemleri. Nobel Yayincilik, 498
- nOzmen, I., Bayir, A., Cengiz, M., Sirkecioglu, A.N., Atamanalp, M., (2004). Effects of wa-ter reuse system on antioxidant enzymes of rainbow trout (Oncorhynchus mykiss), Vet-erinarni Medicina Czech, 49: 373–378
- nPickering, A.D., Pottinger, T.G., (2006). Recov-ery of the brown trout, Salmo trutta L., from acute handling stress: a time-course study, Journal of Fish Biology, 20: 229-244.doi: 10.1111/j.1095-8649.1982.tb03923.x
- nReiter, R., Tang, L., García, J.J., Muñoz-Hoyos, A., (1997). Pharmacological actions of melatonin in oxygen radical pathophysiolo-gy, Life Sciences, 60: 2255-2271.doi: 10.1016/S0024-3205(97)00030-1
- nRonisz, D., Larsson, D.G., Förlin, L., (1999). Seasenol variations in the activities of se-lected hepatic biotransformation and antiox-idant enzymes in celpout (Zoarces vivipa-rus), Comparative Physiology and Biochem-istry, 124: 271-279
- nSanthakumar, M., Balaji, M., Ramudu, K., (1999). Effect of sublethal concentrations of monocrotophos on erythropoietic acticity and certain hematological parameters of fish Anabas testudineus, The Bulletin of Environmental Contamination and Toxicology, 63: 379-384.doi: 10.1007/S001289900991
- nSayeed, I., Parvez, S., Pandey, S., Bin–Hafeez, B., Haque, R., Rausiddin, S., (2003). Oxida-tive stres biomarkers exposure deltame-thrinin freshwater, Ecotoxicology and Envi-ronmental Safety, 56: 295-301
- nSerezli, R., Okumus, I., Akhan, S., (2003). Aqua-culture use of anesthesia, Turkish Journal of Aquatic Life, 475-480
- nShakoori, A.R., Iqbal, M.J., Mughal, A.L., Ali, S.S., (1994). Biochemical changes induced by inorganic mercury on the blood, liver and muscles of freshwater Chinese grass carp, Ctenopharyngodon idella, Journal of Eco-toxicology and Environmental Monitoring, 4: 81-92.doi: 10.1007/s001289900216
- nShakoori, A.R., Mughal, A.L., Iqbal, M.J., (1996). Effects of sublethal doses of fenvala-rate administered continuously for four weeks on the blo, liver and muscles of a freshwater fish, Ctenopharyngodon idella, The Bulletin of Environmental Contamina-tion and Toxicology, 57: 487-494
- nSisecioglu, M., Cankaya, M., Gulcin, I., Ozdemir, M., (2009). The inhibitory effect of propofol on lactoperoxidase, Protein Pep-tide Letter, 16: 46-49.doi: 10.2174/092986609787049394
- nSohlberg, S., Czerwinska, K., Rasmusen, K., So-li, N.E., (1990). Plasma concentration flumequine after intra-arterial and oral ad-ministration to rainbow trout (Salmo gaird-neri) exposed to low water temperatures, Aquaculture, 84: 355-361.doi: 10.1016/0044-8486(90)90100-2
- nTandogan, B., Ulusu, N., (2003). 6-phosphogluconate dehydrogenase: Molecular and kinetic propertyes, Turk Journal of Biochemistry, 28: 268-273
- nUner, N., Oruç, E.O., Canli, M., Sevgiler, Y., (2001). Effects of cypermethrin on antioxi-dant enzyme activities and lipid peroxadition in liver and kidney of the freshwater fish, Oreochromis niloticus and Cyprinus carpio, The Bulletin of Environmental Contamina-tion and Toxicology, 67: 657-664.doi: 10.1007/s00128-001-0174-z
- nYamaguchi, J., Nishimura, M., (1984). Purifica-tion of glyoxymal catalase and immuno-chemical comparison of glyoxysomal and leaf peroxisomal catalase in germinating pumpkin cotyledons, Plant Physiology, 74: 261–267.doi: 10.1104/pp.74.2.261
- nYoshida, Y., Nakano, Y., Yamashita, Y., Coga, T., (1997). The and gene encoding a novel 6PGDH and its adjacent region of Actinoba-cillus aclinomycelemcomitants chrosomal DNA, Biochemical and Biophysical Re-search Communications, 230: 220-225. doi: 10.1006/bbrc.1996.5917











