Keywords
Anguilla anguilla, lead, sera, hematocrit
Introduction
Wide usage of heavy metals in various industries increases their levels in natural ecosystems. Heavy metals are important pollutants affecting all parts of ecosystems since their biological halflives are long and they are being accumulated cumulatively (Bailey et al., 1999).
Lead is not necessary for the biological functions of animals even at low concentrations. It is being discharged to aquatic systems mainly from petroleum, chemistry, dye and mining industries, which has toxic effects and can cause mortality to aquatic animals (Sorensen, 1991; Heath, 1995).
Chronic lead poisoning has similar toxic effects in fish as in mammals. These include hematological and neural disorders and tetanic spasms together with some morphological changes such as darkening in caudal fin, deformation of vertebrate, anomalies in pigment formation and covering of the gills by a mucus layer (Tulasi et al., 1992; Martinez et al., 2004; Shah, 2006).
Hematocrit level is the ratio of blood cells to sera and reflects the oxygen carrying capacity of the blood and functioning of hematopoietic functioning of tissues. Glucose has a high energy capacity and forms the basic source of energy for biological functioning. Sera proteins have a transportation role in carrying various organic and inorganic compounds while cholesterol is a structural component of lipoproteins, bile acids and steroid hormons (Heath, 1995).
Studies carried out with various fish species under laboratory conditions showed that heavy metals cause direct disorders in morphology, osmoregulation and secondary disorders by enzymatic and hormonal changes in sera glucose, protein, cholesterol and blood hematocrit levels (Cicik and Erdem, 1998; Karata? et al., 2005; Arslan et al., 2006).
A.anguilla has a wide ecological valance and is consumed as protein resources. It is important to know the blood parameters to determine the metabolic and physiological state of the fish under the stress of heavy metal pollution since this source of pollution in the freshwaters is rather high. The effect of lead on blood parameters of fish, which forms an important link in the aquatic food chain in freshwater ecosystems, is important in determining the metabolic and physiologic state of the fish. Therefore, the present study was undertaken to determine the effects of lead (0.06 and 0.12 ppm) on sera glucose, protein, cholesterol and on hematocrit levels of A.anguilla.
Material and Methods
Anguill anguilla were obtained from a private aquaculture farm in Silifke, Mersin. Experiments were carried out in Basic Sciences laboratory in Fisheries Faculty under controlled conditions.
Eight glass aquaria (size: 40x120x40 cm) were filled with tap water. Fish were placed in these aquaria and adapted to laboratory conditions for one month. The concentrations of lead used in the experiments were 0.06 and 0.12 ppm which is 5 % and 10 % of the 96h LC50 value (1.17 mg/L) of lead for this species. Experimental periods were carried out in two series (15 and 30 days) and three glass aquaria were used for every series. Two of the aquaria were filled with 120 L of tap water containing lead and used as experimental tanks and the third was filled with tap water without lead as control.
Length and weight of fish were 20.3 ±2.2 cm and 44.4 ±3.1 g respectively. Six fish were placed in each aquarium which is totaly 36 fish for all experiments. Fish were fed with Lumbricus terrestris once a day at amounts of 2% of the total biomass. A central aeration system was used for aeration of the aquaria.
Experimental waters in glass aquaria were replaced every two days due to possible changes in lead concentration caused by sinking and adsorption of lead and evaporation of water.
At the end of 15 and 30 days of experiments fish were transferred to transportation tanks in order to minimize stress which is known to have an important effect on the parameters studied. Fish were anaesthetized with 1.5 ppm Quinaldin (Merck) for 30 minutes. After that they were removed from the tanks, washed with tap water and dried with paper. Blood samples for determining the hematocrit level were taken directly from the heart arteries by decapitating the animals.
Hematocrit levels were determined by microhematocrit methods and sera glucose, protein and cholesterol were measured using “O-Toluidin”, “Quantitative Biurea” and “Wedemeyer and Yasutake” methods respectively (Wedemeyer and Yasutake, 1977).
Statistical analyses of the experimental data were carried out using “Regression Analysis” and “Student Newman Keul’s Procedure” (Sokal and Rholf, 1969).
Results and Discussion
Table 1 shows some of the physical and chemical characteristics of water, such as temperature, total hardsness, total alkalinity, pH and dissolved oxygen.
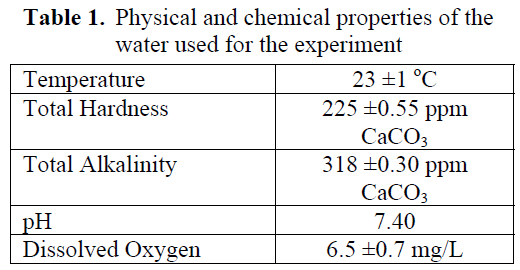
Table 1. Physical and chemical properties of the water used for the experiment
No mortality was observed at both concentrations of the lead studied during the experiments. Some behavioral differences such as sharp movements and avoid taking food was observed at the beginning of the experiments which returned to normal at later stages of exposure.
No statistical difference in hematocrit level was found in fish exposed to 0.12 ppm Pb for 15 and 30 days (P>0.05) while 0.06 ppm Pb caused a significant increase in hematocrit levels at both exposure periods (P<0.05) (Figure 1).
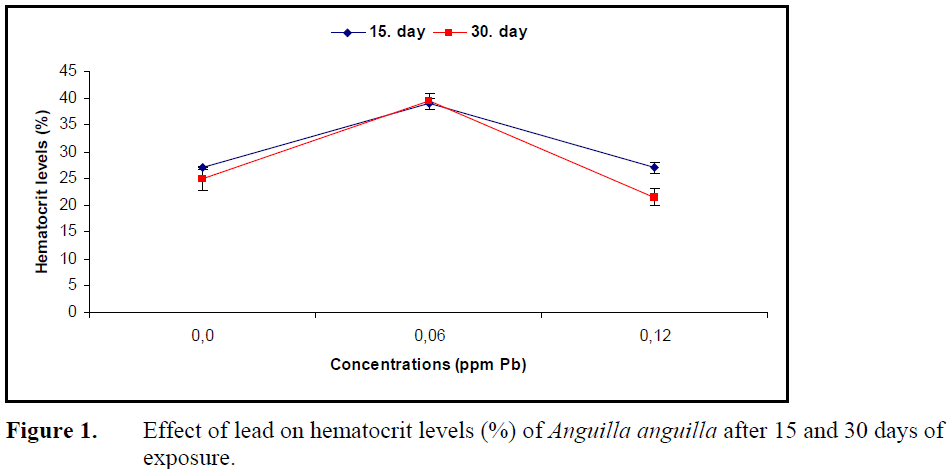
Figure 1. Effect of lead on hematocrit levels (%) of Anguilla anguilla after 15 and 30 days of exposure.
Sera glucose levels increased (P<0.05) (Figure 2) while protein levels decreased significantly (P<0.05) (Figure 3) compared to control at both concentrations of lead.
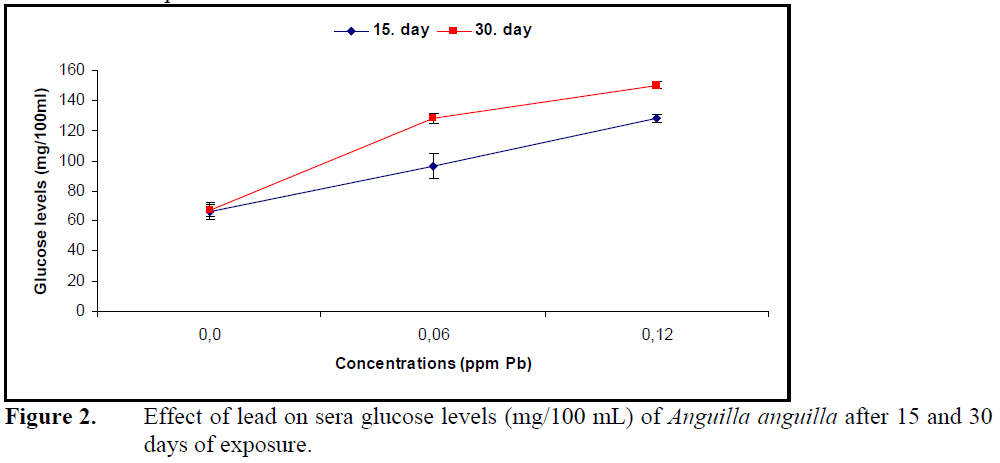
Figure 2. Effect of lead on sera glucose levels (mg/100 mL) of Anguilla anguilla after 15 and 30 days of exposure.
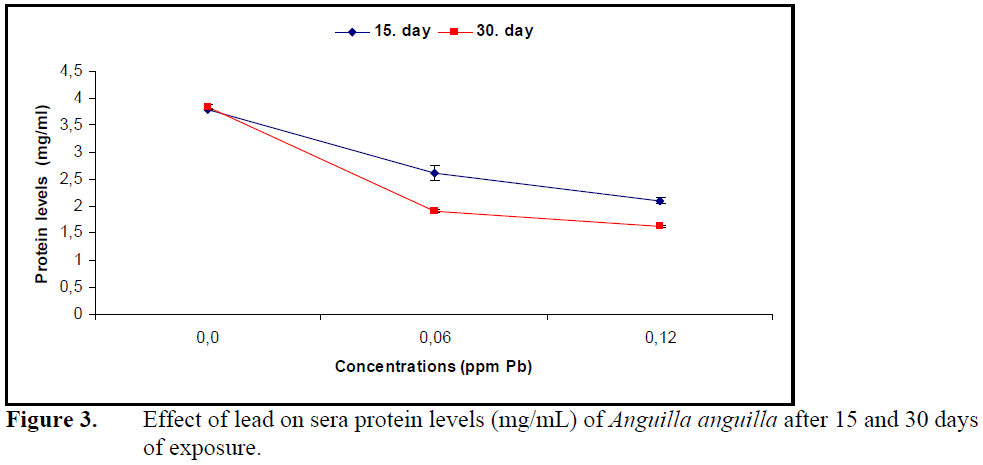
Figure 3. Effect of lead on sera protein levels (mg/mL) of Anguilla anguilla after 15 and 30 days of exposure.
Exposure to both concentrations of lead for 15 and 30 days decreased the cholesterol levels significantly compared to control (P<0.05) (Figure4).
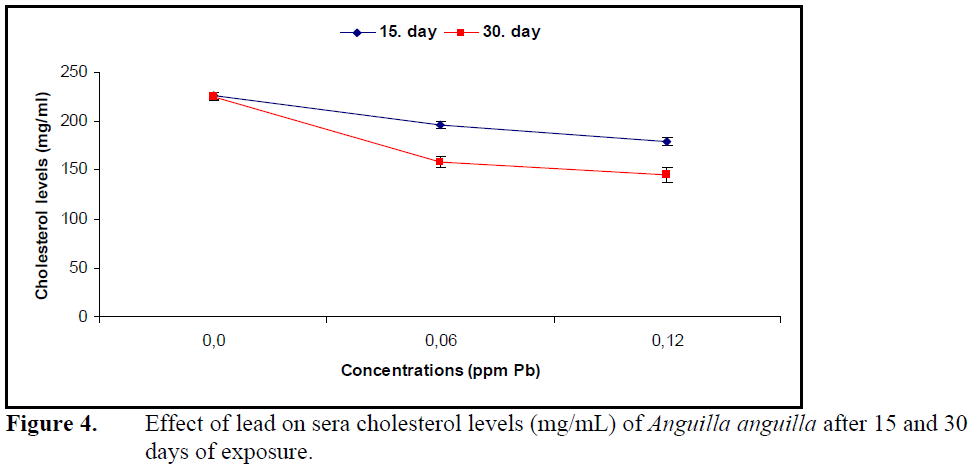
Figure 4. Effect of lead on sera cholesterol levels (mg/mL) of Anguilla anguilla after 15 and 30 days of exposure.
However, the decrease in sera cholesterol levels was not significant between the exposure periods and lead concentrations (P>0.05).
Low concentrations of heavy metals accumulate in different part of the tissues of fish while higher concentrations result in mortality (Heath, 1995). Sublethal concentrations of lead resulted in tissue deformation in Oreochromis niloticus (Cicik et al., 2004), Anabas testudineus (Tulasi et al., 1992) and Tinca tinca (Shah and Alt?nda?, 2005) while exposing to 2.4 ppm Pb for 96 h for Pimephales promelas resulted in 100% mortality (Sorensen, 1991). In this study the concentrations of lead did not cause mortality in A. anguilla after 15 and 30 days of exposure. This might be due to the fact that either lead concentrations was not lethal at an exposure period (30 days) or metal uptake is compensated by detoxification mechanisms of the fish.
Fish respond to changing environmental conditions under the effect of heavy metals by changing their feeding habit, development, breeding and behavioral activities (Almeida et al., 2001). A decrease in swimming performance, an increase in operculum movements and no feeding was observed under the effect of copper in Labeo rohita (Venkataramana and Radhakrishnaiah, 2001), cadmium in Tilapia nilotica (Erdem, 1990) and Cyprinus carpio (Karaytu? et al., 2007) and lead in O.niloticus and T. tinca (Cicik et al., 2004; Shah and Alt?nda?, 2004). These authors reported these behavioral changes to normal after a certain period of exposure to heavy metal . Similar behavioral changes were also observed in A. anguilla under the effect of lead which turned to normal on the later stage of the exposure. These behavioral changes in A.anguilla might be due to stress caused by the metal at first exposure and that metabolic and physiologic change stimulated by detoxification mechanism which might explain the later recovery.
The influence of heavy metals on hemoglobin and hematocrit levels and erythrocyte numbers, which reflects the oxygen carrying capacity of blood, changes depending on the species, type of the metal, its concentration and exposure period. Long term exposure to sublethal levels of copper in C. carpio (Dhanapakiam and Ramasamy, 2001), cadmium in Anguilla rostrata (Gill et al., 1993) and lead in T. tinca (Shah, 2006) resulted in a decrease in hematocrit levels. The reason for this decrease due to hemodilution and osmotic hemolysis under the effect of metal on osmoregulation and membrane permeability. The hematocrit levels increased in Puntius conchonius (Gill and Pant, 1985) under the effect of cadmium and in Prochilodus scorfa under the effect of copper (Mazon et al., 2002) which was explained by an increase in erythrocyte production by erythropoietic tissues and release of erythrocytes from spleen to circulatory system. The decrease in oxygen concentration resulting from the hypoxic conditions under the effect of metal. Hematocrit levels of A.anguilla increased compared to control after exposure to 0.06 ppm Pb over 15 and 30 days, whereas 0.12 ppm Pb did not alter hematocrit levels. The increase in hematocrit levels can be attributed to erythrocyte release by lead stimulation of erythropoietic tissues whereas the stability under the effect of high concentration can be explained by hormonal mechanisms.
Continuation of life in aquatic organisms depends on rebuilding the homeostasis by metabolic and physiologic arrangements under the effect of pollution or changing environmental conditions. Blood is an important component in building homeostasis (Heath, 1995). The effects of heavy metals on biochemical parameters such as sera glucose, protein and cholesterol depend on organism, metal type, exposure concentration and duration. Together with stress factors such as heavy stocking and hunger (Vijayan et al., 1997), effects of cadmium in Heteropneustes fossilis and C.carpio (Sastry and Subhadra, 1982; Cicik and Engin, 2005; Karata? et al., 2005), copper in Salmo trutta and Clarias lazera (Nemcsok and Hughes, 1988; Arslan et al., 2006) and lead in P. lineatus (Martinez et al., 2004) increased the levels of sera glucose and glucocorticoids such as cortisol, epinephrine and chatecholamine. The reason for the increase in sera glucose is suggested to be glucocorticoid induction of glycogenlysis and glycogenesis.
Prolonged exposure to sublethal concentrations of methylmercuric chloride decreased sera cholesterol levels in Lepomis macrochirus and P. lineatus (Dutta and Haghighi, 1986; Martinez et al., 2004). This decrease might be due to hemodilution as a result of improper osmoregulation, inhibition of cholesterol synthesis or its usage in the synthesis of steroid hormones.
Lead in P.lineatus (Martinez at al., 2004) and copper in Ictalurus nebulosus (Christensen et al., 1972) decreased total protein levels as well as sera cholesterol levels. The decrease in sera total proteins was explained by an increase in the synthesis of metal binding proteins. Sera glucose level increased while cholesterol and protein levels decreased under the effect of lead in A.anguilla.
Conclusion
The increase in sera glucose might be a result of excess energy need in metabolic defense against lead and the decrease in cholesterol. Protein levels were probably due to effect of lead on lipid metabolism and to an increase in the synthesis of metal binding protein. It was concluded that lead induced changes in blood parameters of A. anguilla was a consequence of destruction of energy resources in order to maintain homeostasis.
1605
References
- Almeida, J.A., Novelli, E.L.B., Dal-Pai-Silva, M., Alves-Junior, R., (2001). Environmental cadmium exposure and metabolic responses of the Nile tilapia, Oreochromisniloticus, Environmental Pollution, 114 (2): 169-175.
- Arslan, M., Karaytuğ, S., Cicik, B., (2006). Bakırın Clariaslazera (Valenciennes, 1840)’da dokuglikojenve serum glukozdüzeyiüzerineetkileri, Journal of Fisheries and Aquatic Sciences, 23 (1/1): 23-27.
- Bailey, S.E., Olin, T.J., Bricka, R.M. and Adrian, D.D., (1999). A review of potentially lowcost sorbents for heavy metals, Water Researchs, 33: 2469-2479.
- Christensen, G.M., McKim, J.M., Brungs, W.A., Hunt, E.P., (1972). Changes in the blood of the brown bullhead (IctalurusnebulosusLeSueur) following short and long term exposure to copper (II), Toxicology and Applied Pharmacology, 23: 417-427.
- Cicik, B., Erdem, C., (1998). Cyprinuscarpio (L.)’çinko ve bakır + çinko karışımının serum kolesterol derişimini üzerine etkileri, Mersin Üniversitesi, Mühendislik ve Fen Bilimleri Dergisi, 1: 82-87.
- Cicik, B., Ay, Ö.,Karayakar, F., (2004). Effects of lead and cadmium interactions on the metal accumulation in tissues and organs of the Nile tilapia (Oreochromisniloticus), Bulletin Environmental Contamination and Toxicology, 72: 141-148.
- Cicik, B., Engin, K., (2005). The effects of cadmium on levels of glucose in serum and glycogen reserves in the liver and muscle tissues of Cyprinuscarpio (L., 1758), Turkish Journal of Veterinary and Animal Sciences, 29: 113-117
- Dhanapakiam, P., Ramasamy, V.K., (2001). Toxic effects of copper and zinc mixtures on some haematological and biochemical parameters in common carp, Cyprinuscarpio (L.), Journal of Environmental Biology, 22 (2): 105-111.
- Dutta, H.M., Haghighi, A.Z., (1986). Methylmercuric chloride and serum cholesterol level in the bluegill (Lepomismacrochirus), Bulletin Environmental Contamination and Toxicology, 36: 181-185.
- Erdem, C., (1990). Cadmium accumulation in liver, spleen, gill and muscle tissues of Tilapia nilotica (L.), Turkish Journal of Biochemistry, 15: 13-22.
- Gill, T.S., Leitner, G., Porta, S., Epple, A., (1993). Response of plasma cortisol to environmental cadmium in the eel, Anguilla rostrataLeSueur., Comparative Biochemistry and Physiology, 104 (3): 489- 495.
- Gill, T.S., Pant, J.C., (1985). Erythrocytic and leucocytic responses to cadmium poisoning in a freshwater fish PuntiusconchoniusHam., Environmental Research, 30: 327- 337.
- Heath, A.G., (1995). Water pollution and fish physiology, 359, CRC Press Inc. Florida, USA.
- Karataş, S., Erdem, C., Cicik, B., (2005). Kadmiyumun Cyprinus carpio (L.1758)’da serum aspartat aminotransferaz, alanin aminotransferaz ve glukoz düzeyi üzerine etkileri, Ekoloji Çevre Dergisi, 14 (55): 18-23.
- Karaytuğ, S., Erdem, C., Cicik, B., (2007). Accumulation of cadmium in the gill, liver, kidney, spleen, muscle and brain tissues of Cyprinuscarpio, Ekoloji Çevre Dergisi, 16 (63): 16-22
- Martinez, C.B.R., Nagae, M.Y., Zaia, C.T.B.V., Zaia, D.A.M., (2004). Acute morphological and physiological effects of lead in the neotropical fish Prochiloduslineatus, Brazilian Journal of Biology, 64 (4): 797-807.
- Mazon, A.F., Monteiro, E.A.S., Pinheiro, G.H.D., Fernandes, M.N., (2002). Hematological and physiological changes induced by short-term exposure to copper in the freshwater fish, Prochilodusscorfa, Brazilian Journal of Biology, 62 (4a): 621-631.
- Nemcsok, J.G., Hughes, G.M., (1988). The effect of copper sulphate on some biochemical parameters of rainbow trout, Environmental Pollution, 49: 77-85.
- Sastry, K.V., Subhadra, K.M., (1982). Effect of cadmium on some enzyme activities in tissues of the freshwater catfish Heteropneustesfossilis, Toxicology Letters, 14 (1-2): 45-55.
- Shah, S.L., Altındağ, A., (2004). Behavioural abnormalities of tench (Tincatinca L., 1758) on exposure to mercury, cadmium and lead, Fresenius Environmental Bulletin, 13 (12b): 1482-1485.
- Shah, S.L., Altındağ, A., (2005). Effects of heavy metal accumulation on the 96-h LC50 values in tenchTincatinca L., 1758, Turkish Journal of Veterinary and Animal Sciences, 29: 139-144.
- Shah, S.L., (2006). Hematological parameters in tenchTincatinca after short term exposure to lead, Journal of Applied Toxicology, 26: 223-228 Sokal, R.R., Rholf, J.F., (1969). Biometry, 776, W.H. and Freeman Company. San Francisco, USA.
- Sorensen, E.M., (1991). Metal poisoning in fish, 243, CRC Press, Boca Raton, USA. Tulasi, S.J., Reddy, P.U.M., Romano-Rao, J.V., (1992). Accumulation of lead and effects on total lipids on lipid derivatives in the freshwater fish Anabas testudineus (Bloch), Ecotoxicology and Environmental Safety, 23: 33-38.
- Venkataramana, P., Radhakrishnaiah, K., (2001). Copper influenced changes in lactate dehydrogenase and G-6-PDH activities of freshwater teleost, Labeorohita, Archives of Environmental Contamination and Toxicology, 67: 247-263.
- Vijayan, M.M., Pereria, C., Grau, E.G., Iwama, G.K., (1997). Metabolic responses associated with confinement stress in tilapia: the role of cortisol, Comparative Biochemistry and Physiology, 116 (1): 85- 89.
- Wedemeyer, G.A., Yasutake, W.T., (1977). Clinical methods for the assessment of the effects of environmental stress on fish health, United States Technical Papers Fish Wild Services, 89: 1-18.











