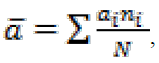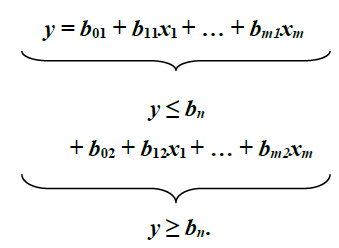Keywords
Brown trout, Stocking brood-fish, Effects on Population Structure, Gene Pool Introgression
Introduction
It was common in the fishery management that human activities posed a strong risk of cer-tain kinds of adverse effects on native stocks, e.g., compromising the original biodiversity and loss of intraspecific variability after an introduc-tion of non-native strains of brown trout and change in genetic composition of their wild stock, i.e., the decrease of the indigenous genetic variability from interbreeding (White, 1989; Ry-man et al., 1995), or disturbance of life-history features that might affect also the fisheries value of native brown trout stock. For the management implications it is important to notice that in many species fitness is affected by physical perfor-mance, which eventually also links to morpholo-gy (Huckins, 1997; Belk et al., 2007). Fish stock-ing is to be considered an important fishery man-agement, as well as conservational activity that is to be accomplished as a way of compensating the shortfalls in recruitment, or in threat of extinction by target species that arises through overfishing in the Catch-and-Remove fishing regime applied, or through environmental degradation. Where fish are stocked into self-reproducing populations of the same species, the effects of stocking is dif-ficult to evaluate, since the understanding the population dynamics is particularly challenging when the populations are supported by a mixture of stocking and natural reproduction. Compensa-tory responses in growth and mortality are likely to reduce the absolute contribution to recruitment from natural stocks and the contribution to repro-duction from stocked fish is often uncertain, es-pecially when the stocked fish are from the strain not adapted to the recipient water (Welcomme, 2001). When they belong to the strain that shows invasive character, they quickly compromise the indigenous character of native stocks by intro-gression, as revealed frequently in both world-wide (Laikre and Ryman, 1996, Laikre et al., 1999) and local fishery management with brown trout Salmo trutta (L., 1758) stocks (Mari? et al., 2006; Razpet et al., 2007). Both for economic and population dynamics issues, the juveniles of hatchery fish have been almost exclusively used for stocking. However, their hatchery produced morphology, probably insufficient for a natural stream environment, the most likely result was poor survival in the wild (Vehanen et al., 2009). Several kinds of impact by stocked brown trout on the native ones were recognized also in the UK (Anonymous, 2003), e.g., competition and predation by stocked fish, stimulation of influx of predators, stimulation of fishing effort and thus an excessive exploitation of wild stocks, as well as the introduction of diseases and change in ge-netic composition of wild stocks due to inter-breeding. The more recently promoted Catch-and-Release fishing regime fundamental to sports in recreational fishery in temperate countries ena-bled easier model of fishery management, alt-hough it can make certain damages from hook setting and releasing that might result in prone-ness to diseases and feeding difficulties, which might lead to the increased mortality rate. This regime showed especially positive effects in con-servation of indigenous stocks. For that reason, it is widely applied in the management with wild trout stocks.
Considering the significance of brown trout stocks in fishery and conservational senses, it is desirable to assess characteristics of brown trout from the fish caught and released unharmed back into its habitat. Sticking to that often makes the investigation of certain characteristics (e.g., re-productive characters) causing damage, or sacri-ficing of fish, very difficult. Since the knowledge of those characteristics is usually necessary for the evaluation of the status of brown trout stocks, it was important to find a way to investigate them to minimize the harmful effects of data collection on brown trout. In accordance with the theory of saltatory ontogeny (Balon, 1975; 1990), shifts from one to the next developmental period have been shown to be followed by more-or-less ab-rupt alteration of various characteristics (e.g., habitat use and morphology) in various fish spe-cies, e.g. in ruffe Gymnocephalus spp. (Ková? 1994), stone loach Barbatula barbatula L. (Ko-vá? et al., 1999) and minnow Phoxinus phoxinus (L.) (Simonovi? et al., 1999). Following that, Simonovi? et al. (2000) demonstrated that abrupt alteration in modes of growth (both in length and in weight) in huchen Hucho hucho (L.), detected by Piecewise Linear Regression (Nickerson et al., 1989), at both smaller (i.e. late juvenile peri-od) and trophy sizes (i.e. late adult period), corre-sponds well to the ages of first maturation, i.e. the onset of adulthood (detected breakpoints at 3.005 kg and 49.6 cm Sl) and of the onset of se-nescence (detected breakpoints at 16.541 kg and at 110.0 cm Sl), respectively. In addition to that, Simonovi? and Nikoli? (2007) revealed that size at which brown trout attain maturity coincides very well with the breakpoint values obtained for resident brown trout from two streams in Serbia, as learnt from the records obtained in two exami-nations accomplished for the prosecuting of poachers. The similarity of Fulton Coefficient (Fc) values for both smaller and larger huchen from the catching reports at the River Drina fish-ing area in the 2005 – 2007 time period to those from the 1998 – 1999 time period and in allome-try occurring for growth –in-length and -in-weight for smaller and larger huchen, respective-ly (Simonovi? et al., 2011), strongly validated the use of Piecewise Linear Regression, i.e., the breakpoints of size-related variables for detection of developmentally-related life-history events in fisheries research. Simonovi? and Nikoli? (2007) reported that resident brown trout in the River Gradac matured at very large size in comparison to the stocks from the smaller streams. They stat-ed that age structure of the population would rise, i.e., the maturation would be attained at greater body size if the river depth was greater at con-stant abundance and biomass of brown trout, or reversely, the maturation would be attained at smaller body size if abundance would rise at con-stant biomass of brown trout, i.e., the age struc-ture of the population would change by drop of average age. So, greater the mean individual length, i.e., the lower the relative density, the maturation would be attained at greater size. Jen-kins et al. (1999) reported the strong negative re-lationship between the individual size and density of brown trout, suggesting that large trout are competitively advantageous in comparison to smaller ones when density increases.
The first aim of this study was to test the null hypothesis (H0) that stocking of brown trout of the brood size did not affect the population struc-ture and life-history features (mean weight, aver-age age, relative abundance, relative biomass and annual natural production, time of maturation) of native brown trout in the River Gradac. Consider-ing the strictly applied Catch-and-Release fishing regime during the whole six-years management period and lack of records about (i.e., negligible rate of) illegal fishing, as well as the approxi-mately same CPUE and structure of brown trout catch, it seems that fisheries pressure and rate of poaching can be excluded as factors that affected the change in population structure in brown trout from the River Gradac, and that stockings, espe-cially that one accomplished with the brood size fish in the distinct, upstream section of the stream, were the events that impacted the change in the population structure of brown trout there.
In the beginning of the management period in 2001, there was no information about the mo-lecular status of brown trout in the River Gradac, yet. Before the stocking events in 2007 (in 2005, when the sampling for the molecular analyses was worked out by fly fishing), it was learnt that brown trout of Atlantic (At) lineage, of the Atcs1 haplotype already occur in the River Gradac in approximately one-third of the population (which should be taken with caution, due to the small number of samples processed) (Mari? et al. 2006). Considering this, the more recent molecu-lar status of brown trout in the River Gradac was also wanted to be ascertained for, in order to check how much brown trout of the At lineage already introgressed into the gene pool there. Hence, in the 2008 sampling, fin clips form only two brown trout were taken, since the majority of brown trout sampled then looked peculiar (Figure 1, A) in compare to both commonly colored fish of the presumably Danubian lineage (Figure 1, B) and those of the strange and strong punctuation featuring brown trout of the Atlantic lineage (Figure 1, C). Considering that no legal justifica-tion for this action was seen at the moment of sampling, it was not possible to take fin clips from more fish.

Figure 1: One (of two) brown trout sampled at the locality D in 2008 for RFLP screening (A) of about 30 cm in Sl, typical brown trout of Danubian line-age of about 25 cm in Sl caught by fly fishing on 4 May 2007(B) and typical brown trout of Atlantic lineage of about 40 cm in Sl caught by fly fish-ing on 9 June 2007(B) (the length of the cork handle of the rod is about 20 cm), both close to the locality M at the upper section of the River Gradac.
Materials and Methods
Study Site and Fisheries Management Plan
River Gradac is a capacious freestone stream situated in the vicinity (about 100 km to south-west) of Belgrade, the capital of Serbia (Western Balkans, south-eastern Europe) (Figure 2). It is about 14 km and of approximately same size all along, due to extensive water capturing from ma-jority of numerous springs that used to feed it. It flows through the picturesque gorge and the whole landscape was proclaimed a natural protected area at early 1990s. The upper section is considered the part from the spring to the Monas-tery ?elije and downstream section down to the dam at the area of Deguri? village is considered the middle section. The size (width, depth and water discharge) of the River Gradac in those two sections is about the same, despite of the nearly 5 km of distance between them, due to the exten-sive water capturing from the springs for the lo-cal water-supplying systems. Occurrence of two dams (one of them being the concrete one, of the height over 10 m) in that section lacking any fish pass facilities makes the downstream migration between brown trout stock much more (if not the only) likely than in the reverse direction. In addi-tion to the scope of brown trout stock fishery management and supplemental to it, the conser-vational scope on the brown trout stock is also important, considering that River Gradac Gorge is the nature protected area obliged by national legislation and specific management documents to maintain indigenous character of animal and plant species.
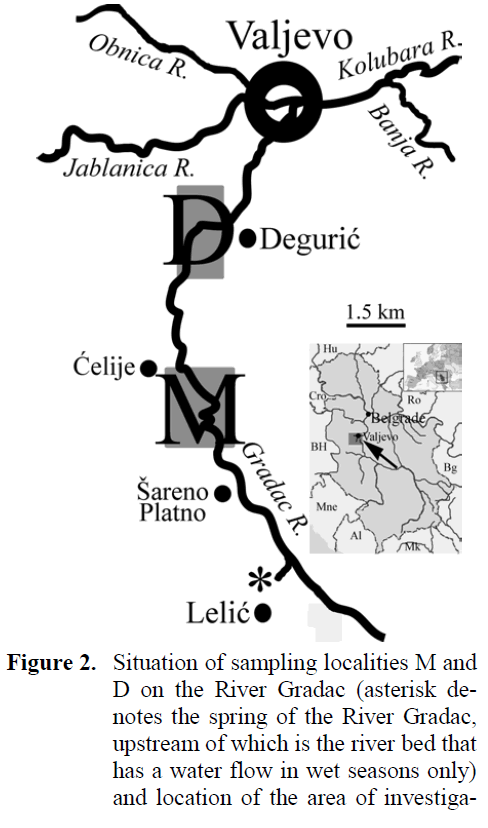
Figure 2: Situation of sampling localities M and D on the River Gradac (asterisk de-notes the spring of the River Gradac, upstream of which is the river bed that has a water flow in wet seasons only) and location of the area of investigation in the region (Serbia) and in Eu-rope.
The last Fisheries Management Plan (FMP) for the River Gradac (Simonovi? et al., 2003) set the main issues for the fishery management in the next five-year time period. It strictly imposed a necessity for the preservation of indigenous brown trout of Danubian (Da) mtDNA lineage (after Bernatchez et al., 1992; Bernatchez, 2001) in the nature protected area where it is situated. Unfortunately, in the time of the preparation of that FMP, it was not known that native brown trout stock in the River Gradac was already com-promised by stocking with the 1+ hatchery reared brown trout of Atlantic lineage in 2001, as re-vealed by Mari? et al.(2006), who detected af-terwards that one out of three brown trout sam-pled from the River Gradac was of the Atcs1 hap-lotype. The FMP also issued the strict “Catch-and-Release" fly fishing of then extremely abun-dant brown trout without any stocking as a pre-cautionary, conservation-related measure that was to minimize a risk of introduction of domesticated, hatchery-reared brown trout strains (e.g., of the Atlantic lineage) that are alien for this area, already used in the closely situated Slovenia for fish stocking for a long time (Mari? et al., 2011).. The possibility of stocking in the FMP was re-stricted only to an occasion of strong spring tor-rents washing out the newly hatched brown trout parr. That fishing regime without additional stocking was enforced until 2007. In the late spring 2007, after the strong public pressure from the fly-fishing community on the fishery manager and without the real need (i.e., without any high spring water level and deleterious effect, but only for public reasons), about 1.8 x 104 hatchery-reared brown trout yearlings, i.e., parr of up to 10 cm in standard length (Sl) were introduced in two stocking occasions along the upper and middle sections of the River Gradac. However, on the second stocking occasion, 102 brown trout of the average weight (w) of about 1 kg, which were the brood fish from the hatchery, were stocked ex-clusively in the upper section of the River Gradac. Stocking with the brood-size brown trout was worked out beyond the directives given in the FMP and legally issued procedure for stock-ing. There was no checking of genetic status of either yearlings, or brood fish that were stocked then, since there was no legally issued obligation for that.
Sampling Methods
Records from the two samplings of brown trout at the River Gradac were compared. The first sampling was accomplished on the prepara-tion of the FMP, in 18 and 19 August 2003, using the engine-powered (2.4KW) electrofishing gear Suzuki-Bosch™ (220V DC, I = 6A max.), and the second was in 16 and 17 July 2008, using the battery-powered portable electrofishing gear AquaTech™ IG200/1® (380 / 600 V DC, I = 15A max.). On both samplings, the same two sam-pling sites were worked out: one in the upper sec-tion, in the area upstream of the Monastery ?elije (here denoted M), and the other in the middle section, in the area of the Deguri? village (here denoted D). At each sampling site, three stream sections that were of the habitat structure (riffle and pool), length (up to 150 m) and time of work-ing (not exceeding 30 minutes), i.e., of the fish-ing effort as similar as possible, were electro-fished for two upstream passes, with stop-nets spread both at starting and ending fishing points. Since brown trout are considered important in both fishery and conservational sense, as well as there was no need either from the point of legisla-tion, or research to take fish samples, they were returned alive to the same stream section they were sampled from.
Data Collecting
Brown trout samples landed during electro-fishing were stored at large mobile plastic con-tainer both held in and filled with the stream wa-ter. At the end of sampling at each section, each fish was measured for its standard length (Sl) us-ing the measuring tape to the nearest millimeter and weight (w) using the digital scale Philips (of accuracy 1 g). Scales for ageing were taken from the left flank above the lateral line and fish were released alive to the stream. Scales were exam-ined using the Carl Zeiss binocular magnifier un-der the 25 times of magnification.
Statistical Analyses
Data on catch-per-unit-of-effort (CPUE) for brown trout and all other fish species from both sampling sites were used to calculate their rela-tive abundance (in ind ha-1 unit), whereas relative biomass and relative annual natural production (in kg ha-1 unit) were calculated for brown trout only, after Ricker (1975), from average values obtained at each sampling site. Mean age of sam-ples () was calculated according to the age structure (i.e., frequency of occurrence of each age) of each sample, following the expression:
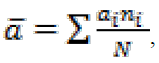
where ai is the age of fish, ni is the frequency of occurrence of that age in the sample and N is the total number of fish of all ages in each sample. Difference in age structure between trout samples from two localities and years of sampling was tested using χ2 test. The speed of growth, both –in Sl and -in w in brown trout samples at two locali-ties in each sampling year was assessed using the linear regression on log-transformed Sl and w. Pairwise tests between samples from different years for their means and regression coefficients were accomplished using t-test.
The investigation of attainment of maturity was accomplished using the Piecewise Linear Regression method of Nickerson et al. (1989) on log-transformed Sl and w, in order to examine whether there is an alteration in growth, i.e., the breakpoint that represents two distinct and signif-icantly different linear regression equations: one for y values less than, or equal to breakpoint, and the other for y values greater than, or equal to breakpoint:
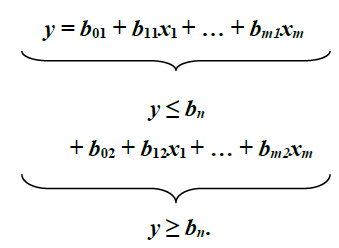
Molecular Analysis
The molecular (i.e., lineage) status after Ber-natchez (2001) of brown trout was ascertained by the method of Restriction Fragment Length Pol-ymorphism (RFLP) of Berg and Farris (1984) from the anal fin clips which were taken from two fishes sampled at the locality D and stored in the 96% ethanol. That small number of samples was due to lack of legal obligation to assess ge-netic status of brown trout and strict limitation to sample fin clips for an analysis that came from the fisheries inspector who surveyed the sam-pling. A total DNA sample was obtained using the High Salt Extraction Technique of Miller et al. (1988). The mtDNA Control Region known also as D-loop (about 1080 bp) was amplified us-ing primers 28RIBa (Snoj et al, 2000) and HN20 (Bernatchez and Danzmann, 1993). PCR was ac-complished under the following conditions: ini-tial denaturation (95°C, 5 min) followed by 30 cycles of strand denaturation (94°C, 45 s), primer annealing (52°C, 45 s) and DNA extension (72°C, 2 min; the last extension prolonged to 5 min) in the MultiGene Thermal Cycler TC9600-G-230V® (Labnet International, Inc.™). Total PCR volume of 20 μl was used, containing 2μl 10μM of each primer (Thermo Scientific™), 0.4 μl 10mM dNTPs (), 1 μl BSA, 2 μl 10 x PCR buffer (Kapa Biosystems™), 0.08 μl 5U/μl Taq polymerase (Kapa Biosystems™) and 1μl (about 100 ng) of genomic DNA. Amplification was checked by loading on the 2% agarose gel and running on 100V for 1 hour. Control Region was afterwards digested using the Thermo Scientific™ FastDigest® SatI endonuclease. Samples for the restriction reaction (10μl PCR amplified Control Region of mtDNA, 2μl 10x digestion buffer, 1 μl, i.e., 10 units of SatI and 17 μl autoclaved distilled water; each in total 30 μl of content) for 25 minutes on 37oC and loaded on the 2% agarose gel with the 0.5 x TBE electrophoresis buffer, with the 25 minutes of staining with the 4μl of Applichem™ SYBR Green® each, in the dark on the room temperature. They were run for 60 minutes at 100V and visualized under the UV light (302 nm). For molecular weight standard, the Thermo Scientific ™ GeneRuler® 50bp ladder was used, together with the three controls of brown trout positively of the Da and At lineages, respectively.
Results and Discussion
The structure of fish community at the locality M appeared significantly different in the 2009 sample in relation to that from the 2003 sample (χ2 = 1951, df = 6, p < 0.01), mainly due to an occurrence of native schneider Alburnoides bipunctatus and chub Leuciscus cephalus as new species for this river section, as well as of trans-located grayling Thymallus thymallus, sparsely (and out of the management plan issues) stocked in 2007 at this section of the River Gradac. In ad-dition to that, the abundance of brook barbel Barbus balcanicus appeared much greater than in 2003 and that of bullhead Cottus gobio was much smaller than in 2003. Significant change in the fish community structure at the locality D in the six year period (χ2 = 350, df = 6, p < 0.01) was mainly due to the appearance of chub and stone loach Barbatula barbatula being the native fish species occurring now at it, with the much less abundance of the rest of native fish species in this river section, except the minnow Phoxinus phox-inus.
The 2003 sample from the site M contained 70 brown trout of range 7 cm to 36 cm in Sl and 4 – 640 g in w, whereas the 2008 sample from that site comprised 25 brown trout of ranges 12 cm to 27.5 cm in Sl and 26 g to 277 g in w. The 2003 sample from the site D contained 11 brown trout of 13 cm to 25.5 cm in Sl and 27 g to 237 g in w, whereas that taken in 2008 held 17 brown trout of 6.5 cm to 35 cm in Sl and 5 g to 634 g in w (Fig-ure 3).
The average age of brown trout increased from 2003 to 2008 (Table 1) significantly only at the locality M (t = 2.815, df = 45, p < 0.01) and not on the locality D (t = 0.591, df = 17), but change in abundance at particular age classes (Figure 3.) resulted in significant difference be-tween age structure of brown trout samples from 2008 and 2003, both on locality M (χ2 = 195, df = 5, p < 0.01) and locality D (χ2 = 150, df = 5, p < 0.01). The first prominent difference in the struc-ture of brown trout population was that at the locality M in 2008 in compare to that in 2003 there was the absence of brown trout yearling (0+). Whereas, that age class was rather abundant at the locality D in 2008, in difference to 2003. The second obvious difference was an occurrence of brown trout of greater size (both -in length and -in weight) and older age at the locality D in 2008, in contrast to 2003 samples.
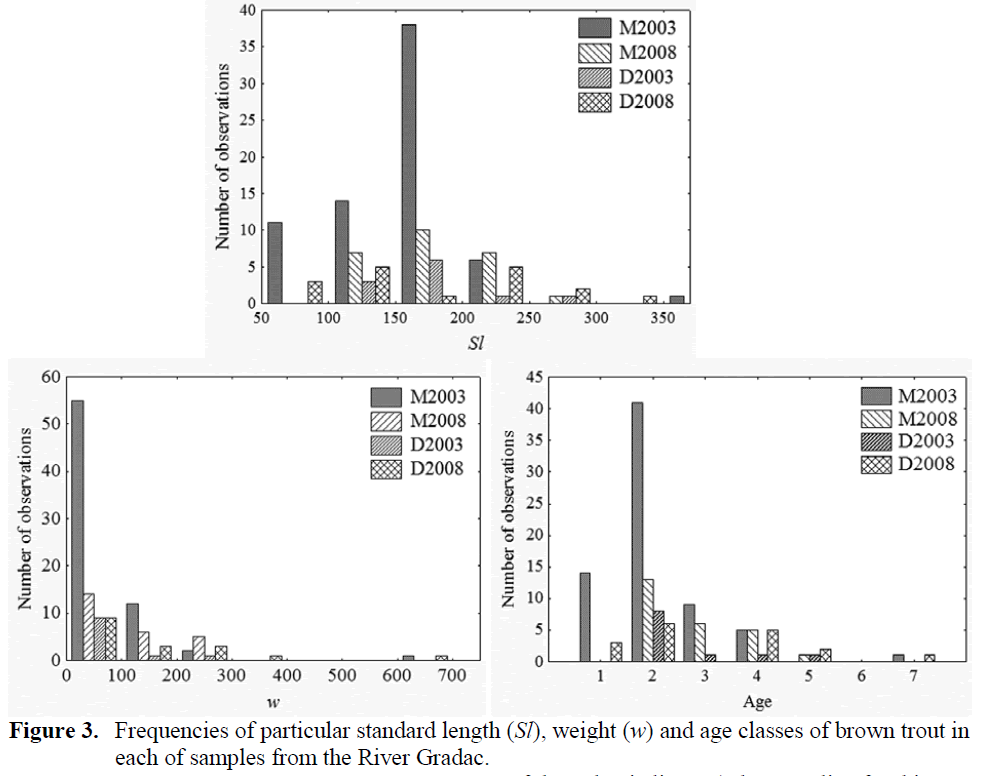
Figure 3: Frequencies of particular standard length (Sl), weight (w) and age classes of brown trout in each of samples from the River Gradac.
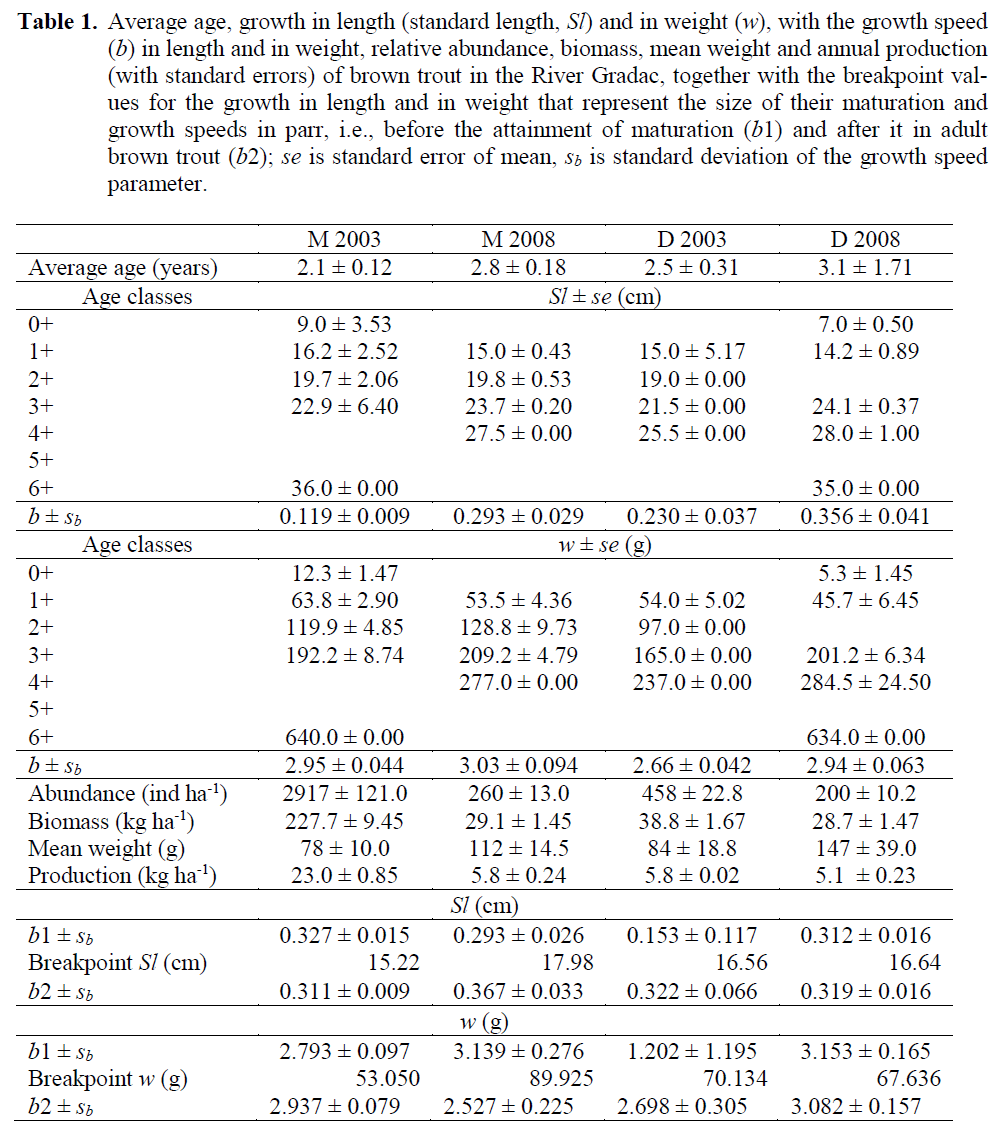
Table 1: Average age, growth in length (standard length, Sl) and in weight (w), with the growth speed (b) in length and in weight, relative abundance, biomass, mean weight and annual production (with standard errors) of brown trout in the River Gradac, together with the breakpoint val-ues for the growth in length and in weight that represent the size of their maturation and growth speeds in parr, i.e., before the attainment of maturation (b1) and after it in adult brown trout (b2); se is standard error of mean, sb is standard deviation of the growth speed parameter.
In compare to brown trout samples from 2003, those from 2008 on both localities showed the decline in relative abundance, relative biomass and annual natural production, as well as the in-crease in mean weight (Table 1). Decline in rela-tive abundance was significant both at localities M (t = 4.364, df = 94, p < 0.01, and D (t = 3.312, df = 26, p < 0.01). The decline in relative bio-mass was significant at locality M (t = 8.894, df = 94, p < 0.01) but not at the locality D (t = 0.941, df = 26, p > 0.05). The decline in annual natural production at the locality M was significant (t = 2.375, df = 94, p < 0.05), and at the locality D it was not significant (t = 1.942, df = 26, p > 0.05). The increase in mean weight was not significant both at localities M (t = 0.303, df = 94, p > 0.05) and D (t = 0.578, df = 26, p > 0.05).
Using average Sl and w values for ages in brown trout samples (Table 1), it appeared that brown trout samples differed for their increased speed of growth in Sl (tSl = 5.730, df = 93, p < 0.05), but not in w (tw = tSl = 0.771, df = 25, p > 0.05) at the locality M. Whereas, the speed of growth at the locality D was not significantly dif-ferent in Sl (tSl = 2.281, df = 93, p < 0.05), alt-hough the growth in w there was significantly faster in brown trout from the 2008 sample (tw = 3.698, df = 25, p < 0.02). Attainment of sexual maturity in brown trout as revealed from break-points occurred at the locality M at greater Sl and w in 2008 than in 2003 (Figure 4), which means either they dominantly matured almost one year later, i.e., in the age of 2+ in 2008, than in 2003, or that only the proportion of the largest 1+ indi-viduals attained the maturity in the same age. At-tainment of maturity at the locality D in 2008 sample occurred at almost identical Sl and slight-ly smaller w than in 2003 sample (Table 1), meaning they matured at approximately the same age of 2+ during the six-year period. Brown trout parr grew in Sl at the locality M almost the same in 2008 as in 2003 (t = 1.133, df = 40, p > 0.05), as well as in w (t = 1.183, df = 40, p > 0.05). Whereas, after they matured, their growth in Sl in the 2008 sample was significantly faster (t = 5.737, df = 53, p < 0.01), although in w it was similar (t = 1.719, df = 53, p > 0.05) when com-pared to that in the mature brown trout from the 2003 sample. In the D sample, the growth of parr was similar in 2008 as in 2003, both in Sl (t = 1.346, df = 15, p > 0.05) and in w (t = 1.617, df = 15, p > 0.05), as well as that of mature brown trout both in Sl (t = 0.176, df =10, p > 0.05) and in w (t = 1.119, df = 10, p > 0.05).
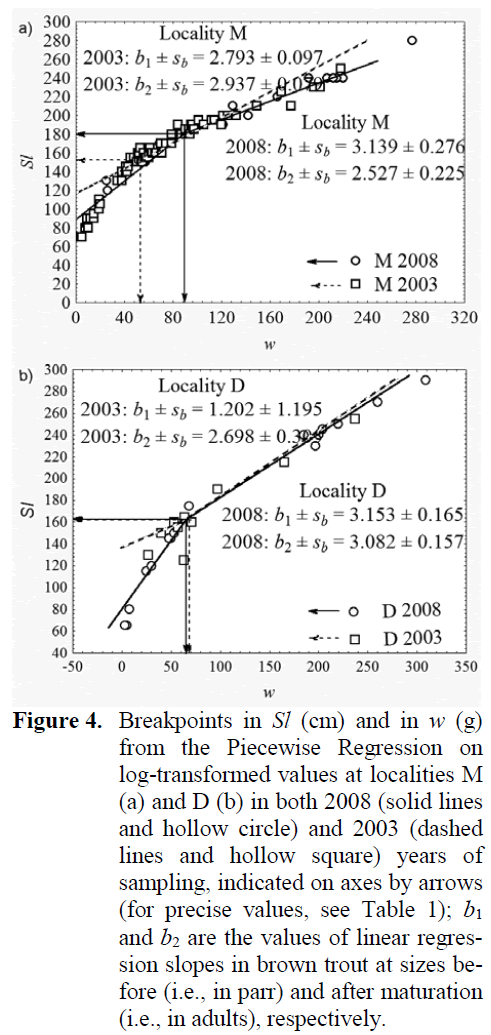
Figure 4: Breakpoints in Sl (cm) and in w (g) from the Piecewise Regression on log-transformed values at localities M (a) and D (b) in both 2008 (solid lines and hollow circle) and 2003 (dashed lines and hollow square) years of sampling, indicated on axes by arrows (for precise values, see Table 1); b1 and b2 are the values of linear regres-sion slopes in brown trout at sizes be-fore (i.e., in parr) and after maturation (i.e., in adults), respectively.
Restriction analysis (RFLP) with the SatI en-donuclease revealed that both fishes from the locality D, of the coloration that was not typical for brown trout of either the Da, or At lineage, were of the At lineage, as revealed from both 50 kb ladder and three positive controls, two of Da and one of the At lineages (Figure 5). The restriction endonuclease split the Control Region only in brown trout of the At lineage at C434 in two parts of the sizes 434bp and 646bp, whereas in those of the Da lineage the Control Region mtDNA re-mained intact (of 1080bp in length).
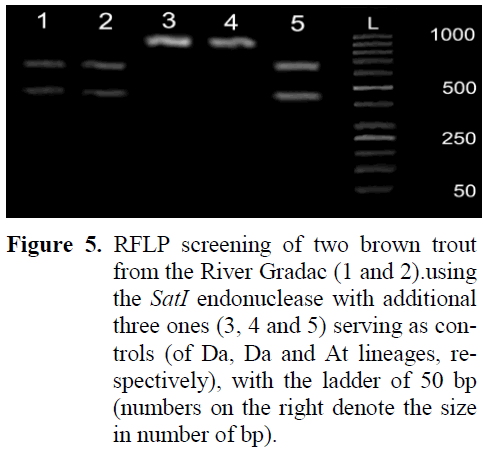
Figure 5: RFLP screening of two brown trout from the River Gradac (1 and 2).using the SatI endonuclease with additional three ones (3, 4 and 5) serving as con-trols (of Da, Da and At lineages, re-spectively), with the ladder of 50 bp (numbers on the right denote the size in number of bp).
Although Welcomme (2001) stated that it is difficult to evaluate the effects of stocking in field when fish are stocked into self-reproducing population of the same species, the difference recorded in the structure of both fish community and brown trout population between years 2003 and 2008 found at one of two investigated locali-ties of the River Gradac coincides with the dif-ference in stocking that occurred there. In order to state unequivocally that differential stocking in the upper and lower section of the River Gradac was the main cause for that, it would be desirable to have the data about the population structure of brown trout and fish community for each year in that period, but there was no such kind of moni-toring accomplished. Instead, there are some observations available from both management activities and fly fishing (e.g., CPUE and structure of brown trout in catches, personal observations). According to the records about the number of redds that were counted each year, there was an increase from less than three hundred in 2003, to almost five hundred in 2006 (pers. comm. with the fishery manager clerk); the records that give the provisional number of redds are due to difficulties in distinguishing the redds that were al-ready counted from the new ones within the cou-ple of days of surveillance), which indicated the strong increase in number of spawning fish dur-ing that time period. The insight into the structure of brown trout catches during the management period suggested the common structure of brown trout population in the River Gradac, with the majority of fish in lower size- and age-classes and the smaller proportion of the large, especially the trophy-size (over 50 cm Sl) fish. That, follow-ing the reports of fly fishermen and their wishes for the trophysized trout, was the driving force of the pressure on the fishery manager for the stocking with the hatchery-reared parr, but also with the brood fish in the upper course (i.e., where the locality here assigned M is situated) that occurred in the spring 2007.
At the upstream situated locality M, where the stocking with both parr and brood-size hatchery-reared brown trout occurred, the decrease in abundance of brown trout and bullhead as fellow members of that type of fish community was coupled with the increase in abundance of chub and schneider as members of the fish community that would be usually expected in the more downstream section. Appearance of chub and schneider in the upper section of the River Gradac, at the locality M is coincident with the drop in density of brown trout that are their spa-tial competitors and predators, respectively. That implies a likely occurrence of the empty room there. Chub inhabited pools, especially their en-trance sections, whereas schneider were situated mainly in the long and calm riffles (glides), where parr of brown trout were absent from, or very rare. Constant abundance of minnow at the locality D corresponded to the much more stable structure of brown trout stock there in compare to that in the upstream section.
The drop of brown trout biomass at the locali-ty M in 2008 was huge despite of stocking, with the strong decrease in abundance, i.e. the fading of 0+ (young-of-the-year) parr (Table 1). Management effects increased significantly the aver-age age of brown trout at that locality, since the proportion of mature brown trout older than 2+ increased in 2008 sample at the locality D, in compare to that recorded in 2003 sample (Figure 3). The occurrence of brown trout of ages 1+ and 2+ in the M2008 sample indicates theirs survival by an attainment of the size-refuge from the pre-dation of older and larger fish that remained in the greater proportion in that sample. The in-crease in average age and in abundance of larger fish in the D2008 sample implies to the shift of those categories from the upstream locality M to the downstream locality D, with the competition between the large sized brown trout as the most likely cause that acted there in addition to the predation on parr. The drop in abundance of the 0+ parr and increase in abundance of older and large-sized brown trout that resulted in an in-crease of average age occurring at the locality M in 2008 are in agreement with the findings of Jenkins et al. (1999) that large trout, whose abundance and density were suddenly augmented there by massive stocking, were competitively advantageous in compare to smaller ones when overall density increased after the brown trout parr were stocked. It would be expected that high density of mature, older and larger brown trout would slow their speed of growth, whereas the consequent drop in density of parr at locality M in 2008 (Table 1) would made possible an in-crease in speed of their growth in Sl. In contrast to expectations, there was no acceleration in growth of parr, but only in mature brown trout, and only at the locality M. That significant in-crease in growth of mature brown trout at the lo-cality M is to be considered a consequence of the richness of parr, which likely were the predomi-nant feeding resource for the stocked brood-size fish and native adult brown trout. The drop in density, i.e., in biomass and abundance and com-plete lack of 0+ parr in the age structure of brown trout at the locality M could be addressed to the strong predation from the heavily stocked brood-size hatchery brown trout (about 100 one-kilogram fish) in the rather short (about 4 km long) river section. The predatory pressure of that brood fish in abundance of about 25 fish km-1, which is approximately one fish per each 40 m, on the parr, even without the resident large brown trout occurring there natively, should be consid-ered heavy. This corresponds to the reports of anglers about the difference in the structure of catches of brown trout, with the abundance of large, but slim brown trout of the weight much lower than it would be expected, as well as the scarcity of smaller brown trout in catches.
Increase in breakpoint values indicating the delay in maturation that followed the decrease of population density (relative abundance) in M 2008 samples (Figure 4) is concordant to the re-ports of Simonovi? and Nikoli? (2007) that breakpoint, i.e., maturation is inversely related to brown trout density. That again justifies the use of the Piecewise Linear Regression method that derives the breakpoint between the growth peri-ods as an approach in fisheries management as non-invasive, reliable and easy for an assessment of size at maturation. Using breakpoints could contribute to the advance in management with trout stocks, e.g., for the setting of legal landing size limit on streams and rivers where trout har-vesting is to be allowed. Breakpoints are also in-sensitive of age-structure, because their value is dependent primarily on the alteration in the speed of growth occurring due to the investment into maturation under the state of the life-history traits in the population, and not due to only age itself. The strong increase in breakpoint values of brown trout at the locality M revealing the delay in maturation corresponded to the significant de-crease in density and was accompanied with the significant increase of average age of brown trout there. At the locality D, where brown trout stock was more stable, the breakpoint values, i.e., the onset of maturation in brown trout remained al-most the same.
Considering that locality M was subjected to the stocking with both parr and brood size brown trout at rather high densities of about one fish per each 4.5 m and one fish per each 40 m of river length, respectively, whereas the locality D was subjected to stocking with only par at density of about one fish per each 4.5 m, the effects record-ed at the locality M are to be addressed mainly to the stocking with the large, brood size brown trout. The strong predatory effect of large, adult brook (Salvelinus fontinalis) and rainbow (On-corhynchus mykkis) trout, especially when in high density, to the yearlings (0+) of both trout species was also reported by Larson and Moore (1985). In addition to the drop in brown trout density, the decrease in annual natural production coupled with the delay in maturation occurring at the locality M of the River Gradac could cause an extension of the brown trout stock recovery peri-od in that river section. In contrast to that, neither the annual natural production, nor the relative bi-omass of brown trout of the locality D dropped. That, together with the significant increase in the speed of growth in w, validated that stocking with parr that was carried out according to the fisher-ies management plan was non-detrimental.
Apart of the evident change in the population structure at the locality M, as well as of the find-ing of brown trout individual of the Atcs1 haplo-type by Mari? et al (2006), there is a direct evi-dence from the locality D that stockings with brown trout definitely introduced brown trout of the Atlantic lineage (Figure 4) into the gene pool of the indigenous stock of the Danubian lineage occurring in the River Gradac (Figure 4). The pe-culiar coloration of body (i.e., both dispersal pat-tern and shape of black and red spots) of brown trout landed at the locality D in 2008 sampling (Figure 4) was dissimilar both from those of Da and At lineages. The RFLP analysis using the SatI endonuclease revealed that both brown trout that were strange in coloration were of the At lin-eage, indicating that stocked brown trout of At lineage has already incorporated into the gene pool of native brown trout of Da lineage at the locality D in the lower section of the River Gradac. Considering that the River Gradac is a protected natural area, there are even more im-portant ecological, scientific, economic, cultural and moral/spiritual reasons (Bosse, 2004) why the conservation and restoration of the native Danubian brown trout strain in it the should be undertaken. In addition to that, it is necessary to take care about both water capturing and de-creased shading from riparian vegetation occur-ring due to timber cutting that cause the rise of summer water temperature, which could compromise the conservation and restoration efforts despite of the “Catch-and-Release” regime pro-scribed. The effective way of restoration of na-tive stock seems the one Mitro (2004) reported an appropriate for wild trout in Wisconsin, USA. That approach was already proposed at the be-ginning of the period of management for the Riv-er Gradac (Simonovi? and Kutonova, 2004), within the frame of activities that were found ap-propriate for incorporation into the overall man-agement with the declared natural protected area. It comprised the revitalization of old water mill(s) that were additionally intended to house provisional hatcheries of native brown trout, due to their infrastructural convenience for that. The lack of financial means disabled implementation of this proposal then and further management ac-tivities additionally burdened the status of the brown trout stock in the River Gradac.
Conclusion
Coincidence in change of the structure of fish community, in change of the stock of brown trout, as well as of brown trout life-history traits (age- and size-structure, abundance, density and time of maturation) that followed the stocking that involved both parr and brood fish at the up-stream locality of the River Gradac, in compare to the lack of such events at the other, down-stream locality where the stocking was accom-plished with parr only, imply that brood fish stocked into the upper section influenced detect-ed changes. The fishery records about the in-creased number of redds in spawning seasons and brown trout size distribution in catches during the five year management period that revealed the stable brown trout stock status support that. Ad-ditional effect of stocking which was recorded is introgression of the Atlantic strain of brown trout into the native stock of Danubian Lineage, as re-vealed from individuals of uncommon coloration.
Acknowledgement
The paper was supported by Grant 173025 of the Ministry of Education and Science of Serbia.
264
References
- Anonymous, (2003). National trout and grayling fisheries strategy. UK Environment Agency, Bristol
- nBalon, E.K., (1975). Terminology of intervals in fish development, Journal of the Fisheries Research Board of Canada, 32: 1663-1670. doi: 10.1139/f75-196
- nBalon, E.K., (1990). Epigenesis of epigeneticist: the development of some alternative con-cepts on the early ontogeny and evolution of fishes, Guelph Ichthyological Reviews, 1: 1–42
- nBelk, M.C., Benson, L.J., Rasmussen, J., Peck S.L., (2008). Hatchery-induced morpholog-ical variation in an endangered fish: a chal-lenge for hatchery-based recovery effects, Canadian Journal of Fisheries and Aquatic Sciences, 65: 401-408. doi: 10.1139/f07-176
- nBerg W.J., Farris S.D., (1984). Restriction endo-nuclease analysis of salmonid mitochondrial DNA, Canadian Journal of Fisheries and Aquatic Sciences, 41: 1041-1047. doi: 10.1139/f84-121
- nBernatchez, L. (2001). The evolutionary history of brown trout (SalmotruttaL.) inferred from phylogeographic, nested clade, and mismatch analyses of mitochondrial DNA variation, Evolution, 55: 351-379
- nBernatchez,m L., Danzmann, R.G., (1993). Con-gruence in control-region sequence and re-striction–site variation in mitochondrial DNA of brook charr (SalvelinusfontinalisMitchill), Molecular Biology and Evolution, 10: 1002-1014
- nBernatchez, L., Guyomard, R., Bonhomme, F., (1992). DNA sequence variation of the mi-tochondrial control region among geograph-ically and morphologically remote European brown trout Salmotruttapopulations, Mo-lecular Ecology, 1: 161-173. doi: 10.1111/j.1365-294X.1992.tb00172.x
- nBosse, S., (2004). In defense of natives: Why protecting and restoring native trout should be our highest management priority. In: Moore, S.E., Carline, R.F., Dillon, J. (Eds.), Wild Trout VIII Symposium “Working To-gether to Ensure the Future of the Wild Trout”, 20 – 22 September, Yellowstone Na-tional Park, 117 – 123
- nHuckins, C.J.F., (1997). Functional linkages among morphology, feeding performance, diet and competitive ability in molluscivo-rous sunfish, Ecology, 78: 2401-2414.doi: 10.1890/0012-9658(1997)078[2401:FLAMFP]2.0.CO;2
- nJenkins, Jr., T.M., Diehl, S., Kratz, K.W., Cooper, S.D., (1999). Effects of population density on individual growth of brown trout in streams, Ecology, 80: 941-956.doi: 10.1890/0012-9658(1999)080[0941:EOPDOI]2.0.CO;2
- nKová?, V., (1994). Early ontogeny of three Gym-nocephalus species (Pisces: Percidae): re-flections on the evolution of the genus, En-vironmental Biology of Fish, 40: 241-253.doi: 10.1007/BF00002511
- nKová?, V., Copp, G.H., Fransis, M.P., (1999). Morphometry of the stone loach Barbatulabarbatula: do mensural characters reflect the species life history thresholds? In: Balon, E.K. (Ed.), Development in Environmental Biology of Fishes 19. Kluwer Academic Publishers, Dodrecht, 105-115
- nLaikre, L., Ryman, N., (1996). Effects on intra-specific biodiversity from harvesting and enhancing natural populations, Ambio, 25: 504-509
- nLaikre, L., Antunes, A., Apostolidis, A., Berrebi, P., Duguid, A., Ferguson, A., Garcia-Marin, H.L., Guyomard, R., Hansen, M.M., Hindar, K., Koljonen, M.-L., Largiarder, C., Mar-tinez, P., Nielsen, E.E., Palm, S., Ruzzant, D., Ryman, N., Tryantaphyllidis, C., (1999). Conservation genetic management of brown trout (Salmotrutta) in Europe. Report by the Concerted action on identification, manage-ment and exploitation of genetic resources in the brown trout (Salmotrutta), "Ttoutcon-cert", EU Fair CT97-3882
- nLarson, G.L., Moore, S.E., (1985). Encroachment of exotic rainbow trout into stream popula-tions of native brook trout in the southern Appalachian Mountains, Transactions of the American Fisheries Society, 114: 195-203.doi: 10.1577/1548-8659(1985)114<195:EOERTI>2.0.CO;2
- nMiller, S.A., Dykes, D.D., Polesky, H.F., (1988). A simple salting out procedure from human nucleated cells, Nucleic Acids Research, 16: 1215.doi: 10.1093/nar/16.3.1215
- nMari?, S., Snoj, A., Nikoli?, V., Simonovi?, P., (2006). Genetic differentiation of trout (Salmo spp.) populations in Serbia ascer-tained using RFLP technique on PCR ampli-fied control region of mitochondrial DNA, ActaVeterinaria, 56: 423-430.doi: 10.2298/AVB0606423M
- nMari?, S., Simonovi?, P., Razpet, A. (2010). Ge-netic characterization of broodstock brown trout from Bled fish-farm, Slovenia, Peri-odicumBiologorum, 112: 145-148
- nMari?, S., Sušnik, S., Simonovi?, P., Snoj, A., (2006). Phylogeographic study of brown trout from Serbia based on mitochondrial DNA control region analysis, Genetics Se-lection Evolution, 38: 411-430.doi: 10.1186/1297-9686-38-4-411
- nMitro, M.G., 2004. Stocking trout of wild parent-age to restore wild populations: An evalua-tion of Wisconsin’s wild trout stocking Pro-gram. In: Moore, S.E., Carline, R.F., Dillon, J. (Eds.), Wild Trout VIII Symposium “Working Together to Ensure the Future of the Wild Trout”, 20 – 22 September, Yel-lowstone National Park, 255-264
- nNickerson, D.M., Facey, D.E., Grossman, G.D., 1989. Estimating physiological thresholds with continuous two-phase regression. Phys-iological Zoology 62: 866–887
- nRazpet, A., Mari?, S., Parapot, T., Nikoli?, V., Simonovi?, P., (2007). Re-evaluation of Salmo data by Gridelli (1936) – description of stocking, hybridization and repopulation in the River So?a basin, Italian Journal of Zoology, 74: 63-70.doi: 10.1080/11250000601090081
- nRicker, W.E., (1975). Computations and Interpre-tation of Biological Statistics of Fish Popu-lations. Bulletin 191. Fisheries Research Board of Canada, Ottawa
- nRyman, N., Utter, F., Laikre, L., (1995). Protec-tion of intraspecific biodiversity of exploited fishes, Reviews in Fish Biology and Fisher-ies, 5: 417-446.doi: 10.1007/BF01103814
- nSimonovi?, P.D., Garner, P., Eastwood, E.A., Ková?, V., Copp, G.H., (1999). Correspond-ence between ontogenetic shifts in morphol-ogy and habitat use in minnow Phoxinusphoxinus (L.), Environmental Biology of Fish, 56:117-128.doi: 10.1023/A:1007541915307
- nSimonovi?, P., Kutonova, T., (2004). The Gradac River: water mills, trout and public aware-ness. In: Asp, R., Sharp, R., Kutonova, T. (Eds.), Freshwater fisheries in Central and Eastern Europe: the challenge of sustainabil-ity, IUCN, Warsaw, 30-32
- nSimonovi?, P., Mari?, S., Nikoli?, V., (2000). Growth characteristics of huchenHuchohu-cho (L.) from Rivers Drina, Una and Sana, ActaBiologicaIugoslavica – Ekologija, 35: 123-126
- nSimonovi?, P., Mari?, S., Nikoli?, V., (2003). Mid-term Fisheries Management Plan for the Fisheries Area in the Extraordinary Fea-tured Landscape „Gradac River Gorge“ Pro-tected Area. Faculty of Biology &Ecologi-cal Society „Gradac“, Belgrade – Valjevo
- nSimonovi?, P.D., Nikoli?, V.P. (2007). Density-dependence of growth characteristics and maturation in stream-dwelling resident brown trout, Salmotrutta, in Serbia, Fisher-iesManaement and Ecology, 14: 1-6.doi: 10.1111/j.1365-2400.2006.00517.x
- nSimonovi?, P.D., Nikoli?, V.P., Toši?, A.D., Mari?, S.P. (2011). Length-weight relation-ship in adult huchenHuchohucho (L., 1758) from Drina River, Serbia, Biologia, 66: 156-159.doi: 10.2478/s11756-010-0135-2
- nSnoj, A., Jug, T., Melki?, E., Sušnik, S., Pohar, J., Dov?, P., Budihna, N., (2000). Mitochon-drial and microsatellite DNA analysis of marble trout in Slovenia, Journal of Fish Bi-ology (Quaderni ETP), 29: 5–11
- nVehanen, T., Huusko, A., Hokki, R., (2009). Competition between hatchery-raised and wild brown trout Salmotrutta in enclosures - do hatchery releases have negative effects on wild populations?, Ecology of Freshwa-ter Fish, 18: 261-268.doi: 10.1111/j.1600-0633.2008.00343.x
- nWelcomme, R.L. (2001). Inland fisheries ecology and management. Fishing New Books, Blackwell Science, Oxford
- nWhite R.J. (1989). We’re going wild: a 30-year transition from hatcheries to habitat. Trout, Special Anniversary Series: 15-49.