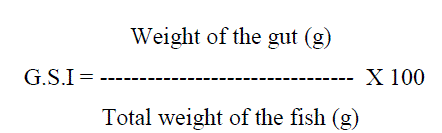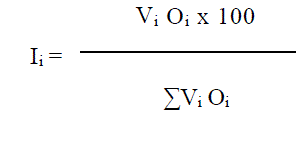Key words
M. aral, RLG, Fullness of gut, GSI, Gut content analysis, Index of pre-ponderance, Upper Assam, India
Özet: Yukar? Assam'dan Yakalanm?? Dikenli Y?lan Bal???n?n Macrognathus aral (Bloch ve Scneider) G?da ve Beslenme Al??kanl???
Macrognathus aral için g?da ve beslenme al??kanl?klar? A?ustos 2008 ve Temmuz 2010 boyunca toplam 421 tane sindirim sisteminin incelenmesiyle yap?ld?. Bal?k yavrular?n?n ortama b?rak?larak stok geli?mesini yürütmek üzere, do?al ortamdaki bal?klar?n beslenme al??kanl???n? bilmek çok önemlidir. Açl???n önlenmesinden dolay? bal?klar?n büyümelerinin geli?mesi ve etkin yönetim bu bilgiler do?rultusunda yap?l?p yönetilebilir. Sonuçlar ortaya koymaktad?r ki ba??rsa??n nispi uzunlu?u (RLG) en yüksek de?erini 7-12 cm’lik grupta 0.62(±0.12) gösterirken, en dü?ük de?eri 27-32 cm’lik grupta 0.58(±0.09) olarak bulundu. Ba??rsak dolu-lu?u; %18.38 (tam dolu); %9.18 (¾ dolu); %11.23 (½ dolu); %12.54 (¼ dolu); %19.77 (nerdeyse bo?a yak?n) ve %28.9 (bo?) olarak kaydedilmi?tir. Gastro somatik indeks (GSI) erkek bireyler için 0.55±0.13 (?ubat)’ den 3.72±1.84 (Haziran)’ e; di?i bireyler için 0.45±0.12 (?ubat)’ den 1.85±0.61 (A?ustos)’ e de?i?im göstermi?tir. Genç bireylerde, kabuklular (%1.97), kumsal ve çamur (%2.05), halkal?larda (%5.38), alg ve bal?k yumurtalar?nda (%17.16), bilinmeyen maddeler içeren ?eyler, böcek larvas?nda (%17.16), zooplankton or-ganizmalar? (%46.44) için güçlü pozitif bir seleksiyon gözlenmi?tir. Yeti?kinlerde en bask?n g?da maddesini mollusc (%5.16), toprak ve çamur (%6.02), crusteceans (%8.02), halkal?lar (%11.75), alg ve bal?k yumurtalar? (%15.04), bilinmeyen maddeleri içeren ?eyler, zooplankton-lar? (%19.56)i takiben böcek larvalar? (%34.45) olu?turmaktad?r. Ön a??rl?k de?erlerinin in-deksi bu türler için en çok tercih edilen g?dan?n böcek larvalar? oldu?unu (%63.1) ve bunu da zooplanktonlar (%20.02) bilinmeyen maddelerden olu?an bile?imler, alg ve bal?k yumurtas? (%10.5), kum ve çamur (%4.37), crustaceans (%1.30), halkal?lar (%0.52) ve molluscs (%0.10)lar?n takip etti?ini göstermi?tir.
Anahtar Kelimeler: M. aral, RLG, Ba??rsak içeri?i, GSI, Ba??rsak içerik analizi, Ön-a??rl?k indeksi, Yukar? Assam, Hindistan
Introduction
Study of food and feeding habits of fishes have manifold importance in fishery biology. For successful fish farming a thorough knowledge about the food and feeding habit is necessary. Fish like any other organisms depends on the en-ergy received from its food to perform its biolog-ical processes such as growth, development, re-production and other metabolic activities. De-tailed data on the diet, feeding ecology and trophic inter-relationship of fishes is fundamental for better understanding of fish life history in-cluding growth, breeding, migration (Bal & Rao, 1984) and the functional role of the different fishes within aquatic ecosystem (Blaber, 1997; Wootton, 1998; Hajisamae et al., 2003). Fish food consumption might be influenced by many environmental factors such as water temperature, food concentration, stocking density, fish size and fish behaviour (Houlihan et al., 2001). As the nature of food depends to a great extent upon the nature of environment, the problem is inter-esting from specific, as well as ecological point of view (Bhuiyan et al., 2006).
The importance of the study of food and feed-ing habits, to evaluate the ecological role of the fish species has been emphasized by many workers. Dutta (1989/1990) studied the food and feed-ing ecology of Mastacembelus armatus (Lecep) from Gadigarh stream of Jammu whereas Sera-juddin & Mustafa (1994) investigated on feeding specialization of adult M. aramatus. Again, Sera-juddin et al. (1998) investigated the food and feeding habits of M. armatus from Kalinadi, a tributary of the Ganga River system at Aligarh. Meanwhile, Ochi et al. (1999) studied on the feeding habit of Caecomastacembelus zebratus in Lake Tanganyika. Further, Serajuddin & Ali (2005) made a study on food and feeding habit of striped spiny eel M. pancalus.
The one-stripe spiny eel, Macrognathus aral (Bloch and Schneider), has been gaining im-portance not only as a food, cultivable fish but also as an aquarium fish for its body shape and behaviour. As per CAMP report (1998) Mac-rognathus aral was included under “Lower Risk near threatened” (LRnt-category). However, Lak-ra & Sarkar (2006) reported M. aral distributed in the Eastern Ghat region of India have found place in the World Conservation Union Red List (2006). Pethiyagoda et al., (2008) quoted that in 1992, an illustrated ‘Wanted’ poster was dis-played by the Wildlife Heritage Trust in the stations of the principal ornamental fish export companies and inland fisheries centres in Sri Lanka, offering a reward of approximately US$ 180 for a single specimen of Macrognathus aral.
Further, detail information on different as-pects of feeding biology of the aforesaid the spe-cies is not available in the north eastern India, which is very vital for sustainable management and conservation of fishery resources. In the north-east India, particularly in upper Assam, the species has very high demand especially when sold alive, fetching a price between Rupees 200 and 280/kg. Without knowledge of the food re-quirements, feeding behaviour pattern, and preda-tor-prey relationships, it is not possible to under-stand the predicted changes that might result from any natural or anthropogenic intervention. Therefore, keeping these in view, a study has been proposed encompassing different aspects of food and feeding habits of M. aral from upper Assam.
Materials and Methods
Food and feeding habits of for M. aral were studied by examining a total of 421 digestive tracts. The specimens of M. aral were collected from different landing stations of lentic and lotic water systems in Dibrugarh and Tinsukia Dis-tricts of Assam between August, 2008 and July, 2010. The guts were taken out from the specimen after measuring and weighing each specimen to the nearest cm and gm respectively and were pre-served in 5% formalin for subsequent analysis. The preserved guts were later uncoiled, cleaned off the attached fat and the length and weight were recorded. The details of the food and feed-ing habits undertaken for the present study are as follows:-
Relative length of the gut (RLG)
The feeding habit was investigated through RLG. The ratio between the gut length and total length (RLG) was estimated by adopting the fol-lowing formula (Al-Hussainy, 1949):- RLG = GL/TL; where, GL stands for total length of the gut and TL are the total length of the fish.
Feeding intensity (GSI)
The feeding intensity of the species in differ-ent life stages and seasons were estimated by ex-amining the fullness of the gut as well as by gas-tro-somatic index (GSI) following the formula (June, 1953; Desai, 1970):-
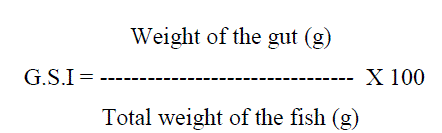
The specimens were properly cleaned in the laboratory and the total length, total weight, sex stage of maturity and degree of fullness were recorded. Degree of fullness of guts were visually classified as full, ¾ full, ½ full, ¼ full, nearly empty and empty depending upon the degree of fullness and the amount of food contained in them (Nwadiaro & Okorie, 1987; Abdelghany, 1993; Bhuiyan et al., 2006). Fishes with full, ¾ full stomachs were considered as active feeders, ½ full as moderate feeders and ¼ full and nearly empty stomachs as poor feeders following the methods used by Rao & Rao (2002) and Raje (2006).
Index of Preponderance
After the collection of the specimens the gen-eral viscera were dissected and the alimentary tracts were separated. For evaluating the im-portance of all food items, the ‘index of prepon-derance’ method (Natarajan & Jhingran, 1961) was employed.
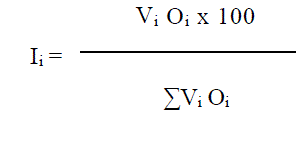
where, Ii = Index of preponderance, Vi and Oi represent the percentage volume and occurrence of particular item of food respectively.
Gut content analysis
Gut content analysis was done for examining seasonal variation in diet components. Further, the data were analysed for different size group to record basic changes in feeding habit. Both vol-umetric and occurrence methods were used for gut content analysis. Both qualitative (volumet-ric) and quantitative (numerical) methods of gut content analysis was employed as recommended by Hynes (1950) and Pillay (1952).
Volumetric method (qualitative)
The content of each sample was taken as a unit and various items are expressed as % volume by eye inspection (Pillay, 1952). The content of each gut was vigorously shaken with distilled wa-ter and then a drop of the content was examined under microscope. The area occupied by each food item was estimated arbitrarily. At least ten such drops were examined and the average of each of the drops was recorded.
Occurrence method (quantitative)
In this method, the number of guts containing a particular item of food was expressed as a per-centage of the total number of gut examined (Hynes, 1950). The method is carried out in two steps-first, all the food items are sorted out and their presence or absence in a particular gut is recorded. Next, the number of guts in which a particular food item present is noted down and the data for all the food item is pooled and con-verted into percentages. This method, apart from describing the qualitative analysis of the diet also gives the frequency of a particular food item oc-curred in the gut and thus helps to understand the preference of any particular food item.
Results and Discussion
Relative length of the gut (RLG) values in M. aral showed little variation among different size groups of all the 421 species examined. The low-est value was found as 0.58(±0.09) in 27-32 cm group (Table 1) whereas the highest values as 0.62(±0.12) in 7-12 cm group. As a whole the RLG value was higher in younger size groups in the species. In this study, RLG of individual spe-cies ranged from 0.54 to 0.68 and was found to be less than 1 as well as remained constant with the increase in length of the fish. The low RLG value shows that the fish falls in the category of carnivorous fishes. RLG value has close relation-ship with the nature of food of the fish (Das & Moitra, 1956). The length of the intestine of the fish depends upon the feeding habits. Carnivores fishes normally have short and more or less straight intestine. This is because the meat gets digested more easily (Pandey & Shukla, 2005; Serajuddin & Ali, 2005) wherein herbivores fish-es the intestine is long and highly coiled because the vegetable food items take more time for di-gestion. The intermediate condition is found om-nivores. Since, M. aral possesses a relatively short and straight intestine, it can be classified as carnivore’s fish.
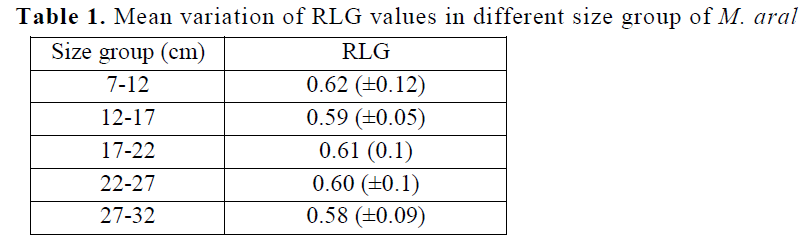
Table 1. Mean variation of RLG values in different size group of M. aral
A percentage occurrence of different degree of fullness of gut for M. aral in different months has been given in Table 2. Out of 421 gut collect-ed over a period of 2 years, it was found that in 78 individuals were full (18.38%), 39 individuals were ¾ full (9.18%), in 47 individuals were ½ full (11.23%), 51 individuals were ¼ full (12.54%), and in 83 individuals were nearly empty (19.77%) and 123 individuals were empty (28.9%). On the other hand, feeding intensity of different maturity stages (Table 3) showed that higher active feeding (full with 28.79% & ¾ full with 10.53%) were found in maturing (stage II); moderate (1/2 full with 29.55%) were found in immature fish (stage II); highest percentage of poor feeding (1/4 full-15.11% and nearly empty-15.11%) were recorded in ripe fish (stage IV). More empty stomachs (76.19%) were found in spent (stage V) than in ripe (69.78%), mature (42.4%), maturing (13.02%) and immature (12.89), which might be due to physiological strain of maturity and spawning.
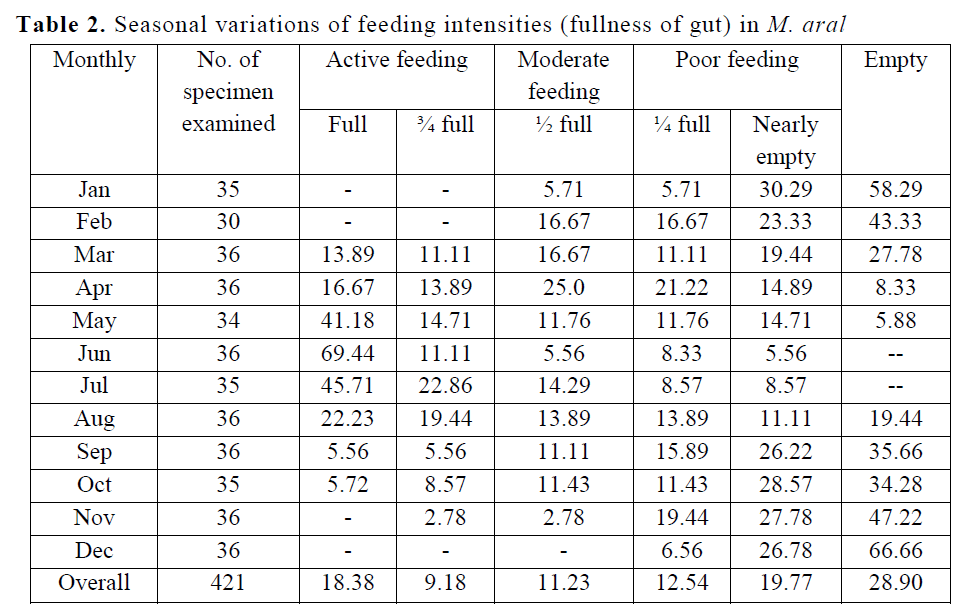
Table 2. Seasonal variations of feeding intensities (fullness of gut) in M. aral
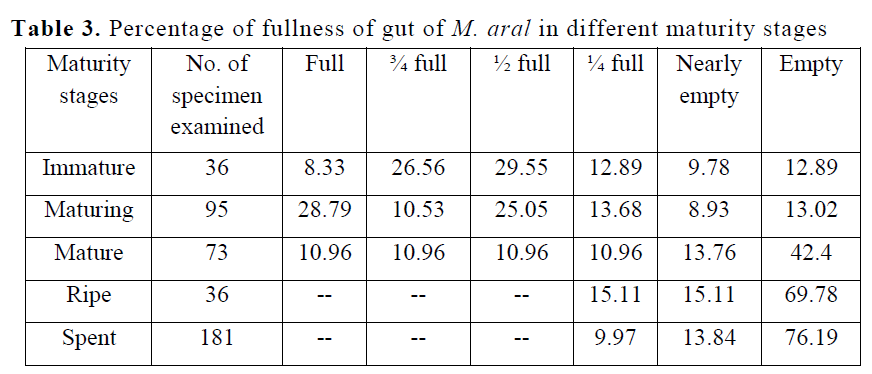
Table 3. Percentage of fullness of gut of M. aral in different maturity stages
Active feeding (full and ¾ full) intensity was high during monsoon (Jun-Aug) with peak in June with a total of 107 species, moderate (1/2 full) during pre-monsoon (Mar-May) with 106 species and poor (1/4 full and nearly empty) and empty during post monsoon (Sep-Nov) and win-ter seasons (Dec-Feb) with 107 and 101 species respectively (Figure 1). The degree of fullness of guts in spiny eel was found to vary with season as well as advancement of gonads. The intensity of active feeding was high during monsoon and moderate feeding observed during pre-monsoon. The poor feeding was mostly recorded in post-monsoon season and it might be due to occur-rence of more number of spawning individuals during this period. In the species, the high per-centage of occurrence of empty stomachs during September to March with peaks in December in-dicates a period of low feeding which also coin-cide low water temperature and non-availability of adequate amount of preferred food items.
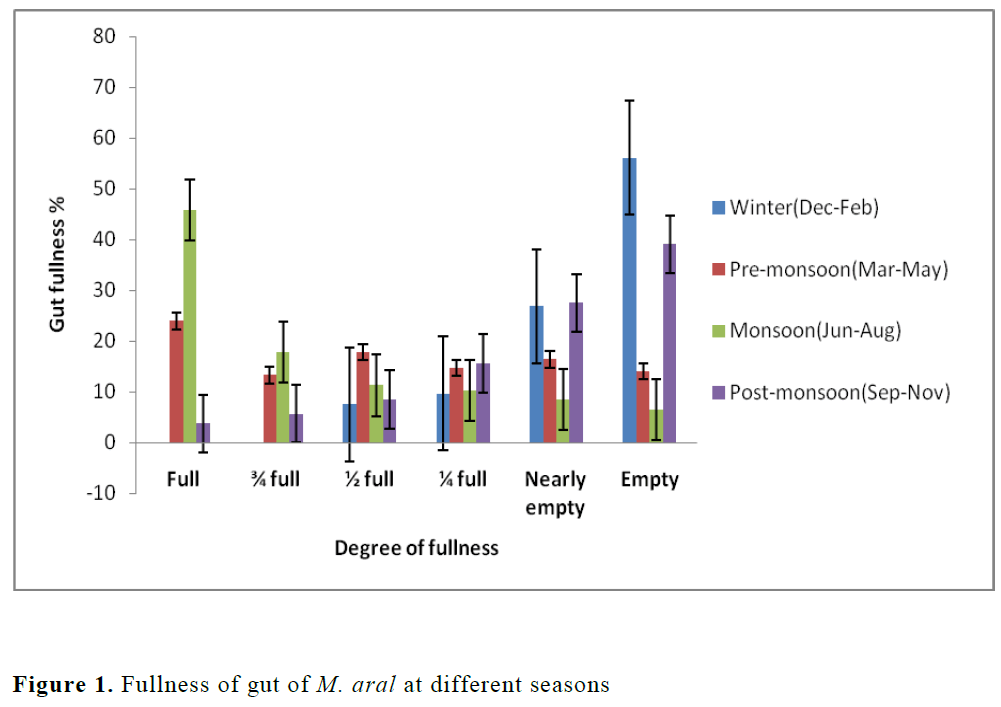
Figure 1. Fullness of gut of M. aral at different seasons
It is no wonder that a large number of fishes were found with empty stomachs in spent speci-mens. The frequent occurrences of empty stom-achs or stomachs with little contents may be probably dependent on the ratio between the size of the fish and size of the prey as cited by Allen (1935) or on the calorific value of the diet as ex-plained by Longhurst (1957) i.e., where fish is an important food item, the daily intake will be less, because of the higher calorific value of the diet and as such empty stomachs will be more com-mon.
As far as gastro somatic index (GSI) in rela-tion to months and seasonal variation for both sexes was concerned, the average monthly GSI of M. aral was ranged from 0.55±0.13 (Feb) to 3.72±1.84 (Jun) for males and that of female from 0.45±0.12 (Feb) to 1.85±0.61 (Aug). GSI was relatively high from May to November (Table 4) with the peak being in June (males) and in August (females) indicating active feeding peri-ods. From November onwards the GSI values of both the sexes declined steadily. As a whole, feeding was better in males throughout the year than females in M. aral. Again, lower GSI for both the males (0.57±0.19) and females (0.54±0.24) of M. aral was observed during win-ter (Dec-Feb) and higher value was observed for males (2.37±0.89) and females (1.38±0.54) dur-ing monsoon (Jun-Aug).
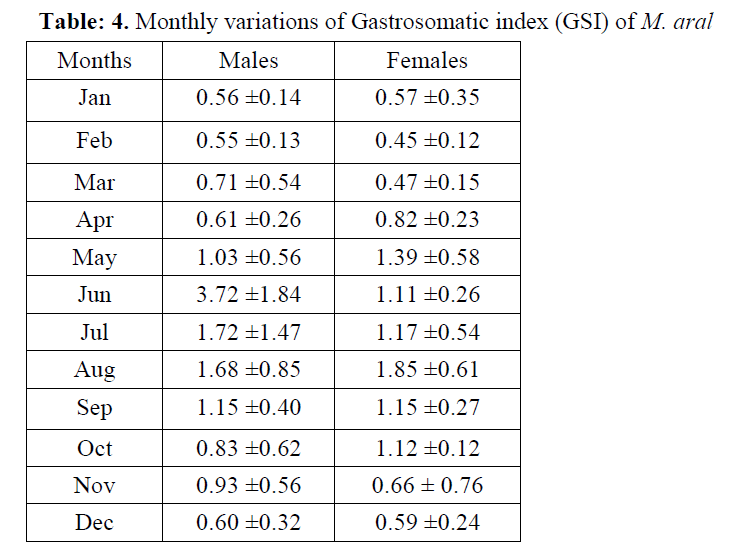
Table: 4. Monthly variations of Gastrosomatic index (GSI) of M. aral
Low feeding activity in female of M. aral might be due to longer spawning period and large number of males in gestating conditions observed in the present study. Mojumdar (1969) and Mo-jumdar & Dan (1981) also reported low feeding intensity in Tachysurus thalassinus and T. tenu-ispinis during their breeding cycle. It may be be-cause more intensive sexual stress in females than that of males as observed by Khumar & Sid-diqui (1989). Serajuddin & Ali (2005) also re-ported less intense feeding during October-December in the species they studied. The feed-ing intensity was better in monsoon and it is in conformity with the observations made by Ven-kataraman (1960). Slight difference was observed in the seasonal feeding intensity of the two sexes. The intensity of feeding in males was higher than in females for M. aral (Figure 2). It may be relat-ed to food abundance during this season and to predominance of immature and maturing fishes which feed actively. Distinctive decline in feed-ing activity for the species during winter (Dec-Feb) can be attributed to recovering stages of gonads as well as low temperature of water and non availability of preferred food. The intake of food subject to variations of preferred food items from season to season. The variation to a large extent seems to be connected with breeding sea-son of the fishes.
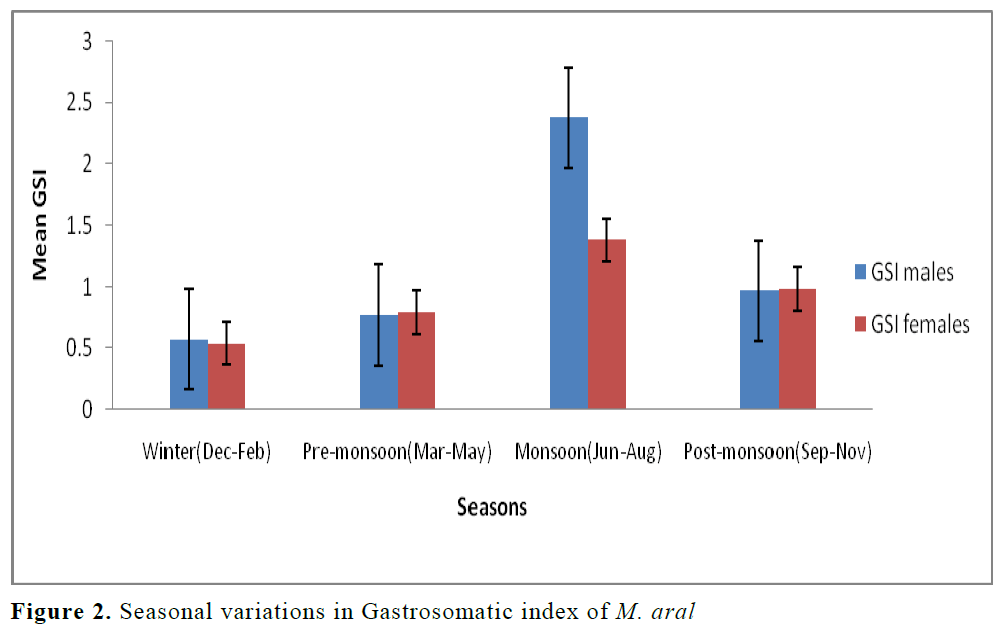
Figure 2. Seasonal variations in Gastrosomatic index of M. aral
Again, GSI in relation to maturity stages, the minimum GSI (0.81±0.34) was observed in spent stage (stage V) and that of maximum (1.86±0.75) in immature (stage I) stage for male M. aral on the other hand, minimum (0.54±0.14) and maxi-mum (1.17±0.45) values were recorded in spent (stage V) and maturing stage (stage II) respec-tively for female M. aral (Table 5). This suggests that, at maturing stage, the fish feed more vora-ciously because of a higher energy demand asso-ciated with gonadal development. Khan (1988) and Serajuddin et al. (1998) also reported the same type of feeding intensity in relation to the stage of maturity in the freshwater catfish, Mys-tus nemurus and spiny eel, Mastacembelus ar-matus respectively.
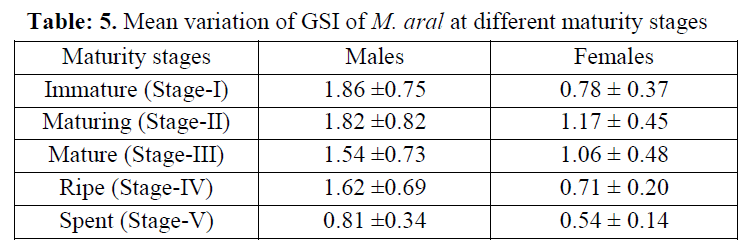
Table: 5. Mean variation of GSI of M. aral at different maturity stages
The intensity of feeding declined when fish became mature/ripe and were ready for spawning and completely reduced to its lowest level in spent fish. The stress brought to bear on the ali-mentary canal of the fish by its developed gonads appeared to be the causative factor in the decline in feeding. The occurrence of poor feeding in other fishes coinciding with peak breeding was reported by various workers (Bhatnagar & Karamchandani 1970; Wijeyaratna & Costa 1987; Khan, 1988). Further, the low feeding ac-tivities in case of spent fishes coincides with the long spawning season. Khan (1972) and Chater-jee (1974) also reported that fluctuation in feed-ing intensity in the fishes took place due to matu-ration of their gonads. In present study too, the feeding activity of spiny eel was found to be fluc-tuated with season as well as maturity stages.
The index of pre-ponderance values (Table 6) of M. aral showed that insect larvae were the most preferred food item (63.1%) for this species, followed by zooplankton (20.02%) miscellaneous includes unidentified matter, algal & fish egg (10.59%), sand & mud (4.37%), crustaceans (1.30%), annelids (0.52%) and molluscs (0.10%). In the index of pre-ponderance, the occurrence of a high percentage of insect larvae followed by zooplankton, and other micro invertebrate fauna in the diet of the species and it revealed that the spiny eel is a carnivorous feeder and feeds in the bottom. Differences in the dominance of different food categories can be attributed to their availa-bility and the habitat where the fish lived at a par-ticular time. According to Desai (1992) food and feeding habits of fish vary as per availability of food, depending on the ecology of the environ-ment. The diet of fish depends on the availability of food which in turn is governed by the biotic factors of the environment. Dutta (1989/1990) and Serajuddin et al. (1998) found aquatic in-sects, crustaceans, annelids, and small forage fish as major food items and indicated stenophagism feeding habit of the species. Similar results were also reported for the closely related species, M. armatus by Serajuddin & Ali (2005). Although the basic feeding habit was almost identical, the intake of different food items showed variation in the present from those of Serajuddin & Ali (2005) and Suresh et al. (2006).
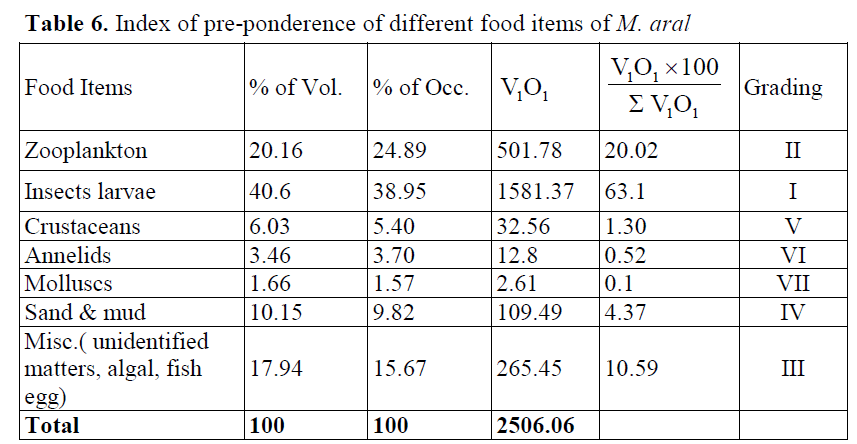
Table 6. Index of pre-ponderence of different food items of M. aral
Gut content analysis reveals that the per-centage composition of food items in the gut of spiny eel as observed in different months has been summarized in the Table 7. The food items found in the examined stomachs were grouped into 7 broad categories viz. zooplankton, insect larvae, crustaceans, annelids, molluscs, sand & mud and miscellaneous includes unidentified matter, algal & fish egg. It was seen that there were considerable variations in the percentage of different food items during different months of the year.
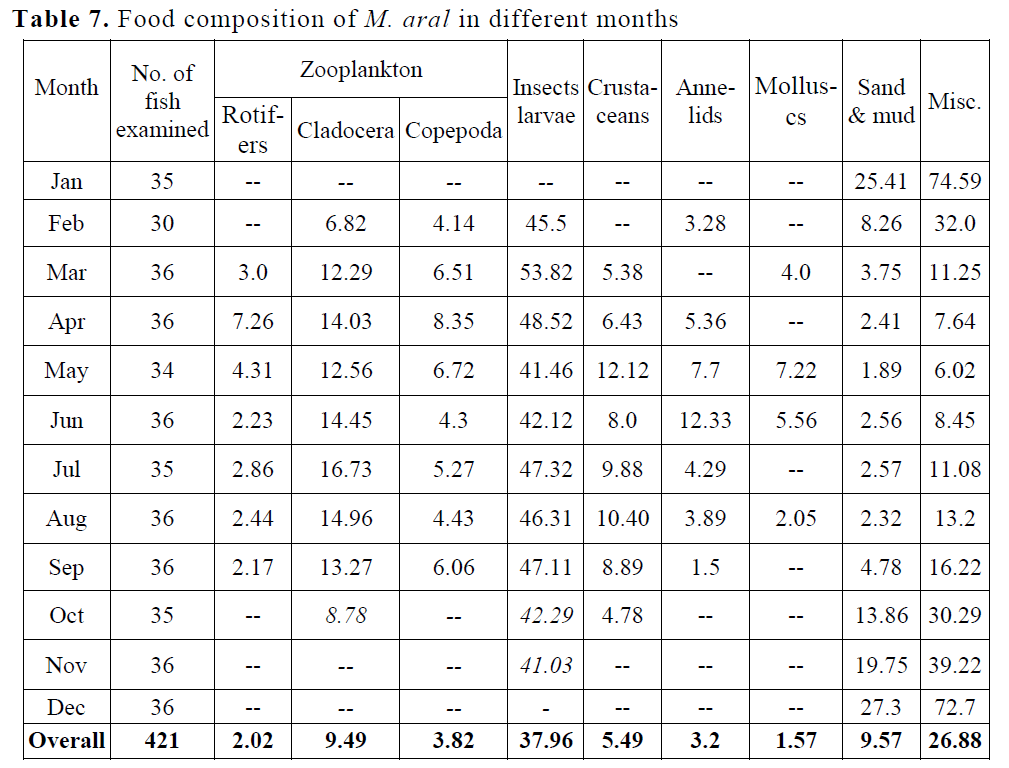
Table 7. Food composition of M. aral in different months
Zooplankton: The highest percentage occurrence of zooplankton was encountered in April (29.64) and lowest (8.78) in Oc-tober
Insect larvae: This item varies from 41.03 (No-vember) to 53.82 (March).
Crustaceans: The highest percentage of Crusta-ceans was observed in May (12.12) and lowest in October (4.78).
Annelids: The percentage occurrence of Annelids was highest in June (12.33) and low-est in September (1.5)
Molluscs: The highest percentage of molluscs occurred in May (7.22) and lowest in August (2.05)
Sand & Mud: The highest percentage of occur-rence of sand & mud in December (27.3) and lowest in May (1.89).
Miscellaneous (includes unidentified matter, al-gal & fish egg): The highest percent-age was observed in January (74.59) and lowest in May (6.02).
Gut content analysis and occurrence of food item reveals that different types of zooplankton were encountered in relatively large quantities in the stomach content of the species during March to October. They were scarce in the gut from No-vember to February but as a whole, contributing to an average of 15.33 % of total food intake in M. aral. Miscellaneous item include unidentified matter, algal and fish eggs were the next pre-ferred food item in the species and annually con-tributed 26.88 %. Insect larvae were present moderately in most of the months except Decem-ber and January and contributed annually 37.96 %. Large amount of sand and mud in the gut of the species were found in October to February and contributing annually as 9.57%. Sand and mud could be taken while fish was burrowing the muddy bottom to prey upon benthic animals. Presence of crustacean in the stomach content in different months showed considerable variation with relatively high percentage of occurrence during March to October. They constituted as 5.49% in the gut content of M. aral. Similarly, annelids and molluscs were also not observed in their gut regularly. The average contribution of annelid formed 3.2 % and molluscs as 1.57% of the total intake of food by these species. The per-centage of molluscs was very low than other food items in throughout the year.
The gut content analysis predicts that the rela-tive occurrence of different food items varied from month to month. Such variation appeared due to varied production or supply of the food items in the environment. Reddy (1991) reported the presence of sand and mud in the stomach of Silago sihama from Karwar waters. The occur-rence of sand and mud throughout the year in the gut confirmed the bottom feeding habit of the fish (Blaber, 2000). According to Moyle & Cech (2000), fishes are classified as deteritivores, her-bivores, carnivores and omnivores based on the type of food. Within these categories they can be further characterized as euryphagous (eating mixed diet), stenophagous (eating limited as-sortment of food types) and monophagous (con-suming only one sort of food). Thus, spiny eel can be classified as euryphagous carnivores with a wide range prey selection. Seasonal change in temperature not only influence food consumption and rate of digestion but also quality and quantity of available food organisms (Bhuiyan et al., 2006).
As far as food composition in different life stages of spiny eel was concerned (Table 8), in juveniles of M. aral, a strong positive selection was observed for zooplanktonic organism (46.44%), insect larvae (27%), miscellaneous in-cludes unidentified matter, algal & fish egg (17.16%), annelids (5.38%), sand & mud (2.05%) and crustaceans (1.97%). The most preferred zo-oplanktonic organisms were rotifers, cladocerans and copepods. In adults of M. aral, the most dominant food item was insect larvae (34.45%) and followed by zooplankton (19.56%) and mis-cellaneous includes unidentified matter, algal & fish egg (15.04%). Annelids (11.75%), crusta-ceans (8.02%), sand & mud (6.02%) and molluscs (5.16) formed the rest of the food items in the guts of the M. aral.
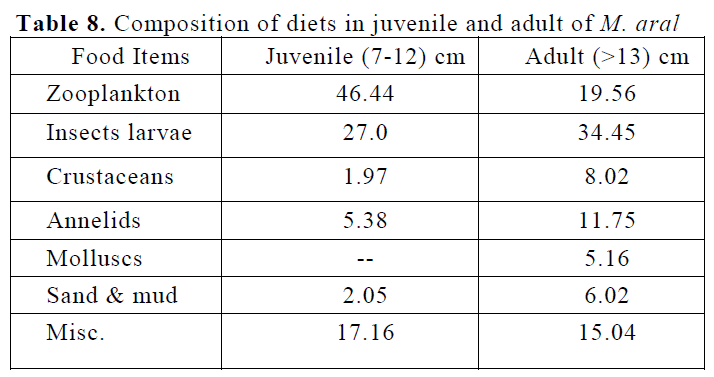
Table 8. Composition of diets in juvenile and adult of M. aral
The gut content analysis of the species in ju-venile and adult fishes shows that a little varia-tion in different types of its food items, with the increase in its length. The zooplankton were the most preferred food item in juvenile of the spe-cies and an insect larvae was observed to be the most preferred food in the adults of the spiny eel. Juveniles were found to consume mostly rotifers, cladocera, copepod etc. Among zooplankton Ke-retella, Monostylla, Rotaria, Daphnia, Alona, Moina, Cyclop, Diaptomus and Eucyclops were preferred by the juveniles while aquatic insect larvae (e.g. Diptera, Hemiptera) and miscellane-ous item (unidentified matter, algal & fish) were eaten mostly by the adult spiny eel. Annelids and crustaceans together represented the secondary food for adults. Molluscs were totally avoided by the juveniles in the species.
The food composition in different life stages of M. aral reveals that the zooplankton were the most preferred food item in juvenile and an insect larvae was being preferred food in adult. Ni-kolsky (1963) suggested that variation in the composition of the food with age and size is a substantial adaptation towards increasing the range of food supply of population by enabling the species as a whole to assimilate a variety of food. The fry and fingerlings of major carps were also found zooplankton as the dominant food (Khan & Siddiqui, 1973). The fishes are known to change their feeding habit as they grow (Ajayi, 1972; Ikusemiju & Olaniyan, 1977). Dutta (1989/1990) while carried out stomach content analysis of M. armatus, reported it as selective insectivorous fish. The present study may be concluded that the occurrence of different types of the food items in gut contents of the fish in different months depend on their availability rather than selection by the spiny eel. The species is a non migratory fish and remains in a specified habitat throughout its life and has to adopt the food available in the habitat during all seasons of the year. The same species occupy different habi-tat may feed on different types of food (Hyndes et al., 1997) or even in the same habitat the diet may vary at different times. The diets of most fish species changes with age and growth. The time and extent of changes in food and feeding habits varies from species to species and often with changes in the style or habitat (Blaber, 2000).
Conclusion
The results reveal that the food and feeding habit of M. aral vary from season to season. The feeding habit of spiny eel was almost identical in juvenile and adult stages. The juvenile and adults of the species feed on higher percentage of zoo-plankton and insects and lower percentage of an-nelids, crustaceans, molluscs, algal materials, fish egg and semi-digested materials etc. The degree of fullness of guts exhibited more or less similar trend in the species. The active feeding was found during monsoon, moderate in pre-monsoon and poor feeding in post monsoon months. It was also observed that the maximum numbers of empty stomachs were recorded during winter season. Again, the poor feeding intensity (GSI) was recorded in spent stage (winter months) while the active feeding intensity was observed in immature and maturing stages (monsoon months). High percentage of sand and mud in the gut indicated that the species is bottom feeder and carnivorous in feeding habit. The diversity and flexibility of food component in the diet ob-served in this study from wild population of M. aral indicates that the fish appears capable of widening the food spectrum in response to habitat availability. Further investigation are required on feeding strategies, diet preferences, and compati-bility with other cultivable fish species during different life stages before adapting in to the cul-ture system.
Acknowledgements
The authors are grateful to the Ministry of Environment & Forests, Govt. of India and also to the Department of Life Sciences, Dibrugarh University, Assam for providing necessary facili-ties to carry out the work.
425
References
- nAbdelghany, E.A., (1993). Food and feeding hab-its of Nile tilapia from the Nile River at Cai-ro, Egypt. pp. 447-453. In: L. Reintertsen, A. Dahle, C. Jorgensen, R. Twinnereim (eds.), Fish Farm Technology, A. A. Balkema, Rotterdam, The Netherland.
- nAjayi, O.O., (1972). Biological studies on the family Bagridae (Pisces: Siluroidei) in Lake Kainji, Nigeria. M. Phil. Thesis, Univ. Ife, Nigeria, pp. 150.
- nAl-Hussany, A.H., (1949). On the functional morphology of the alimentary tract of some fishes in relation to differences in their feed-ing habits, Quarterly Journal of Microscopi-cal Science, 9(2): 190-240.
- nAllen, K.R., (1935). The food and migration of thee perch (Percafluviatilis) in Windermere, Journal of Animal Ecology, 4: 264-273. doi: 10.2307/1016
- nBal, D.V., Rao, K.V., (1984). Marine fisheries of India. Tata McGraw Hill, New Delhi, pp. 296.
- nBhatnagar, G.K., Karamchandani, S.J., (1970). Food and feeding habits of Labeofimbriatus(Bloch) in river Narbada near Hoshangabad (M. P.), Journal of Inland Fisheries Society of India, 2: 30-50.
- nBhuiyan, A.S., Afroz, S., Zaman, T., (2006). Food and feeding habit of the juvenile and adult snakehead Channapunctatus(Bloch), Journal of Life and Earth Sciences, 1(2): 53-54.
- nBlaber, S.J.M., (1997). Fish and Fisheries of Tropical Estuaries, Champan and Hall, London, pp. 388.
- nBlaber, S.J.M., (2000). Tropical Estuarine Fish-es: Ecology, Exploitation and Conservation; Blackwell Science Ltd.; pp. 372. doi: 10.1002/9780470694985
- nChatterjee, A., (1974). Studies on the biology of some carps. Ph.D. Thesis, Aligarh Muslim University, Aligarh.
- nConservation Assessment and Management Plan (CAMP) Report. (1998). Freshwater Fishes of India, National Bureau Fish Genetic Re-source, Lucknow and Zoo Outreach Organi-zation Coimbatore, pp. 327
- nDas, S.M., Moitra, S.K., (1956). Studies on the food of some common fishes of Uttar Pra-desh, India, Proceedings of the National Academy of Sciences India Part II, 26: 213-223.
- nDesai, V.R., (1970). Studies on the fishery and biology of Tor tor(Ham.) from River Nar-bada, Journal of Inland Fisheries Society of India, 2: 101-112.
- nDesai, V.R., (1992). Food of Tor tor(Hamilton) from four localities of India-A comparative analysis, Punjab Fisheries Bulletin, 16(1): 45-48.
- nDutta, S.P.S., (1989/1990). Food and feeding ecology of Mastacembelusarmatus(Lecep) from Gadigarh stream, Jammu. Matsya, 15(16): 66-69.
- nHajisamae, S., Chou, L.M., Ibrahim, S., (2003). Feeding Habits and Trophic Organization of the Fish Community in Shallow Waters of an Impacted Tropical Habitat, Estuarine, Coastal and Shelf Science, 58: 89-98. doi: 10.1016/S0272-7714(03)00062-3
- nHoulihan, D., Boujard, T., Jobling, M., (2001). Food Intake in Fish. Blackwell Science, Ox-ford, UK, 130-143. doi: 10.1002/9780470999516
- nHyndes, G.A., Platell, M.E., Potter, I.C., (1997). Relationships between diet and body size, mouth morphology, habitat and movements of six Sillaginid species in Coastal water: Implications for resource partitioning, Ma-rine Biology, 128: 585-598. doi: 10.1007/s002270050125
- nHynes, H.B.N., (1950). The food of freshwater sticklebacks (Gasterosteusaculeatusand Pygosteuspungitius) with a review of meth-ods used in studies of the food of fishes, Journal of animal Ecology, 19: 36-58. doi: 10.2307/1570
- nIkusemiju, K., Olaniyan, C.I.O., (1977). The food and feeding habits of the catfishes, Chry-sichthys walker (Gunther), C. filamentosus(Boulenger) and C. nigrodigitatus(Lacepede) in the Lekki lagoon, Nigeria, Journal of Fish Biology, 10: 105-112. doi: 10.1111/j.1095-8649.1977.tb04047.x
- nJune, F.C., (1953). Spawning of yellow fin tuna in Hawaiian waters, Fishery Bulletin, 54: 47-64.
- nKhan, R.A., (1972). Studies on the biology of some important major carps. Ph.D. Thesis, Aligarh Muslim University, Aligarh.
- nKhan, M.A., (1988). Biology of Labeocalbasu(Ham.) from Tilaiya reservoir Bihar, Length-weight relationship, condition index and feeding habits, Proceedings of the Na-tional Academy of Sciences India, India, 58B(1): 41-47.
- nKhan, R.A., Siddiqui, A.Q., (1973). Studies on the age and growth of rohu, Labeorohita(Ham.) from a pond (Moat) and rivers Gan-ga and Yamuna, Proceedings of the Indian National Science Academy, 39(3): 582-597.
- nKhumar, F., Siddiqui, M.S., (1989). Food and feeding habits of the carp LabeocalbasuHam. In. North Indian waters, ACTA, Ich-thyolgicaEtPiscatoria, 19: 33-48.
- nLakra, W.S., Sarkar, U.K., (2006). Evaluation of fish biodiversity of Eastern Ghats region for conservation and Sustainable utilization, EPTRI-ENVIS Newsletter, 12 (3): 2-7.
- nLonghurst, A.R., (1957). The food of demersal fish of a West African estuary, Journal of Animal Ecology, 26: 369-387. doi: 10.2307/1753
- nMojumdar, P., (1969). Food of Tachysurusthalassinus(Ruppell), Indian Journal of Fisheries, 16(1&2): 161-169.
- nMojumdar, P., Dan, S.S., (1981). Studies on food and feeding habit of catfish, Tachysurus ten-uispinis(Day), Indian Journal of Fisheries, 26(1&2): 115-124.
- nMoyle, P.B., Cech, J.J., (2000). Reproduction. In Fishes: An Introduction to Ichthyology, 4th Edn., Prentice Hall Inc, 123-144.
- nNatarajan, A.V., Jhingran, A.G., (1961). Index of preponderance – a method of grading the food elements in the stomach analysis of fishes, Indian Journal of Fisheries, 8(1): 54-59.
- nNikolsky, G.V., (1963). Ecology of Fishes. Aca-demic Press, London and New York, pp.352
- nNwadiaro, C., Okorie, P., (1987). Feeding habits of the African Bagrid, Chrysichthysfilamen-tousin a Nigerian Lake, Japanese Journal of Ichthyology, 33(4): 376-383.
- nOchi, Y., Sato, Y., Yanagisawa, Y., (1999). Obli-gate feeding of cichlid eggs by Caecomasta-cembeluszebratusin Lake Tanganyika, Journal of Fish Biology, 54: 450-459.
- nPandey, K., Shukla, J.P., (2005). Fish and Fisher-ies; Rastogi Publications, Meerut; pp.504.
- nPethiyagoda, R., Silva, A., Maduwage, K., Kari-yawasam, L., (2008). The Sri Lanka spiny eel, Macrognathuspentophthalamos(Tele-ostei: Mastacembelidae), and its enigmatic decline, Zootaxa, 37-48.
- nPillay, T.V.R., (1952). A preliminary biometric study of certain populations of Hilsa, Hilsahilsa(HamProceedings of the Indo-Pacific Fisheries Council, Section II, S 2/8.
- nRaje, S.G., (2006). Some aspects of biology of catfishes Tachysuruscaelatus(Valenci-ennes) and Osteogeneiosusmilitaris(Lin-naeus) from Mumbai, Indian Journal of Fisheries, 53(3): 333-340.
- nRao, L.M., Rao, P.S., (2002). Food and Feeding Habits of Glossogobiusgiurisfrom Gosthani Estuary, Indian Journal of Fisheries, 49: 35-40.
- nReddy, C.R., (1991). Some Biological Aspect of Sillagosihamafrom Karwar Waters; A Ph. D. Thesis Submitted to the Karnataka Uni-versity
- nSerajuddin, M., Ali, R., (2005). Food and feeding habits of striped spiny eel, Macrognathuspancalus(Ham.), Indian Journal of Fisher-ies, 52(1): 81-86.
- nSerajuddin, M., Mustafa, S., (1994). Feeding spe-cialization in adult spiny eel, Mastacembelusarmatus, Asian Fisheries Science, 7: 63-65.
- nSerajuddin, M., Khan, A.A., Mustafa, S., (1998). Food and feeding habits of the spiny eel, Mastacembelusarmatus, Asian Fisheries Science, 11: 271-278.
- nSuresh, V.R., Biswas, B.K., Vinci, G.K., Mitra, K., Mukherjee, A., (2006). Biology and fishery of barred spiny eel, MacrognathuspancalusHamilton, ACTA IchthyologicaEtPiscatoria, 36(1): 31-37.
- nVenkataraman, G., (1960). Studies on the food and feeding relationships of the inshore fish-es off Calicut on the Malabar Coast, Indian Journal of Fisheries, 7(2): 274-306.
- nWijeyaratna, M.J.S., Costa, H.H., (1987). The food, feeding and reproduction of the borneo mullet, Liza macrolepis(Smith) in a coastal estuary in Sri Lanka, Indian Journal of Fisheries, 34: 283-291.
- nWootton, R.J., (1998). Ecology of Teleost Fishes. 2ndedition. London: Kluwer Academic Pub-lishers. Fish and Fisheries Series no 24.
- nWorld Conservation Union, IUCN (International Union for Conservation of Nature and Natu-ral Resources). (2006). IUCN Red List of Threatened Species. https://www.iucnredlist.org