Keywords
Galeoides decadactylus; Benin; Gill net; Sex-ratio; Growth parameters; Mortality
Introduction
Fishing is an important activity in the world because it is staple instrument in human’s subsistence (Ekouala, 2013). In Benin, the fishery’s sector contributed a lot in the national economy (Agrer, 2011). But, since some years, the Maritime’s production has decreased (Direction des pêches, 2006; 2012).
This production’s drop is due to an over exploitation of fisheries resources. In fact, the overcapacity of fish flotilla observed in the world (Greboval, 1999), had led progressively an overexploitation, a depletion of fish stock, and the degradation of fish ecosystems ((Kaiser et al., 2002; Adité, 2002; Dulvy et al., 2003; Mullon et al., 2005; Chikou, 2006; Sossoukpe et al., 2013, 2016). This observation was noticed in Benin’s continental fisheries in 1999 by Gbaguidi and Fiogbe (1999).
The overexploitation is due in the one hand to multiplication of sedentary fishery infrastructures (dam, acadjas, whedos) and in the other hand to utilization of devastating engines. This is expressed by a situation of strong regression of capture day by day in spite of increasing of fishery efforts and engine performances (Laleye, 1995; Leveque and Paugy, 1999).
One of the non-selective fishery’s gears in Benin is the Gill net locally named “Soovi” because its mesh sizes are small (30 -40 mm) (Kebe et al., 1997). With this net, the most caught species are: Ilisha africana, Pseudotolithus senegalensis, and Galeoides decadactylus. G. decdactylus of Polynemidae family is one of high value and much sold species (Anato, 1996; Fiogbe et al., 2003).
G. decdactylus is well distributed from the south of Mauritania to Angola (Sidibe, 2010) including the nearshore waters of Benin. This species is well appreciated by the consumers because of the quality of its flesh (Fischer et al., 1981). Its body is fusiform, greyish with two dorsal fins widely speared. The caudal fin is large and forked, and the pectoral one is inserted above the lateral line; the mouth short and infer (Feltes, 1993; Edema and Osagiede, 2011).
This species lives in muddy bottom of coastal zone and often in the estuaries and lagoons (Domain, 1989). The physiological needs of lesser African threadfin caused seasonal migrations in function of its reproduction (Samba, 1974; Lopez, 1978; Domain, 1979) and the cycle of its favorite preys (Konan et al., 2012; Villanueva, 2004).
The African lesser threadfin (G. decdactylus) is a carnivorous and vulture species (Ezekiel et al., 2013). It feed shellfish, little fish, and crabs (Longhurst, 1957; Le Loeuf and Intes, 1973; Samba, 1974; Aggrey-Fynn et al., 2013; Ezekiel et al., 2013).
Since these twenty past years, the lesser African threadfin’s production has been decreasing with more small-sized fishes appearing in catches, and little is known about the species population dynamics. Therefore, the aim of this work was to investigate growth, mortality parameters and exploitation rate of the African lesser threadfin off Benin’s nearshore waters for conservation and management measures.
Material and Methods
Study area, fish sampling and data collection
The study was performed using specimens of Galeoides decadactylus sampled from Gill net hauls landed at the artisanal fishing port of Cotonou (Figure 1). The type of gill net considered is constituted by nets with 30 - 40 mm mesh size. Specimens are sampled twice a week during twelve months (August 2014 to July 2015). Approximately 40% of the total biomass of Galeoides decadactylus (1027 specimens) was sampled in the commercial catches. A total of 956 specimens were sexed. The samples were conveyed in thermos cool boxes to the laboratory.
Figure 1: Location of the Sampling Site.
The total length (TL) of each specimen was measured to the nearest millimeter from the tip of the anterior or part of the mouth to the extremity of the caudal fin. Fish total weight (W) was measured to the nearest 0.1 g after blot drying with a clean hand towel. The specimens were dissected and the internal organs were removed. The gender was determined by macroscopic examination of gonads (King, 1995). The gutted body weight (We) was measured to the nearest 0.1 g. Length data were pooled monthly and converted into length frequencies with a constant class interval of 2 cm. The mean lengths and weights of the classes were used for data analysis using the format accepted by FiSAT (Gayanilo and Pauly, 1997).
Population structure and sex ratio
The size frequency was analyzed at a 2 cm interval total length class using a histogram to determine the type of distribution which characterizes the fish population.
The fluctuations of the sex ratio according to fish size provide a useful tool to examine the biological characteristics of the fish species, such as sexual inversion, longevity in relation to sex, vulnerability to fishing gear and the spatial, seasonal and even daily distribution of species. With the numerical abundance by sex, the following ratio was computed (Anato, 1999):
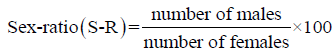
Length –weight relationship
The commonly used relationship W = aTLb was applied (Ricker, 1975) to establish the length–weight relationship by sex, where W is the total weight (W, g), TL is the standard length (TL, cm) and “a” and “b” are intercept and slope of the regression curve of the length and weight of the fish, respectively.
The comparison of the slope “b” to 3 was done with student’s t-test in Minitab software version 16 to establish whether specimen of G. decadactylus grew isometrically.
Growth and mortality parameters
Estimation of growth parameters is essential in order to determine the stock state. The model of von Bertalanffy (1938) is the most used in studies of fish’s growth:

Where, TL (cm) is the total length of the fish, L∞ (cm) is the asymptotic length of fish. It is the maximum length that a fish could have if it lived many years, K (yr-1) is the growth coefficient, t0 (yr) is the theoretical age of fish when his length is zero. It was determined by the Pauly’s equation:
1n(-t0) = -0.392 - 0.275ln L∞ -1.038ln K (Pauly, 1979),
where t (yr) is the age of fish at various lengths and calculated by the inverse of von Bertalanffy growth equation.
Pauly and Munro (1984) proved that for fishes whose growth followed the von Bertalanffy equation, the growth parameters K and L∞ were computed by the relation:
'= ln K + 2ln L∞ ,
where φ' is growth performance index. This index is an indicator of fish’s well-being.
The total mortality (Z) had been estimated by realization of linearized length-converted catch curves (Sparre and Venema, 1992). Instantaneous natural mortality rates (M), were determined by using the Pauly’s (1980) empirical equation:
lnM = −0.0066 − 0.279ln L∞ + 0.6543ln K + 0.463lnT ,
with T the annual temperature of Benin nearshore waters (T = 29.5°C). The fishing mortality (F) rate was determined by the formula:
F = Z – M
First capture length and capture probability
The first capture length was estimated by the software FiSAT. It gives a clear indication on the size of fishes that can be caught by the fishing gear. The probability of capture gave an indication on real size of fish in fishing area that might be caught by the Gill net “Soovi”. This probability was an extrapolation of the length converted catch curve.
Longevity (Tmax)
The formula of Anato (1980) was used: Tmax = 3 / K
Exploitation rate
This rate was given by FiSAT from linearized length converted catch curve of each species. It was compared to the optimum criterion of 0.5 (Pauly and Munro, 1984).
Results
A total of 1027 specimens of G. decadactylus were examined comprising 701 males, 255 females and 71 unsexed. The size range was 10.3 cm - 31.1 cm, and the weigh range was 19.6 g - 327.8 g. The distribution for both sexes was unimodal (16 cm-18 cm) (Figures 2-4). Small sized specimens were more numerous and represent 70% of the total population. Males were greatly more numerous than females. The sex ratio for the studied population was 1:0.36 meaning 100 males for 36 females (Table 1). The length–weight regressions for G. decadactylus are reported in Table 2. The corresponding relationship graphs are presented in Figures 5-7. There is no significant difference between males and females in the length–weight regressions (p > 0.05). The total weight (W) was strongly correlated with total length (TL) (r2 = 0.95). The growth parameters which are the asymptotic length (L∞), the growth coefficient (K), the theoretical age at length zero (t0), and the growth performance index (φ’) were estimated by FiSAT and presented in Table 3. Von Bertalanffy’s function equation is  , where TL is total length and t is the fish age at a moment. The mortality parameters of G. decadactylus fishing by Gill net “Soovi” in the nearshore waters off Benin are presented in Figure 8. The annual instantaneous total mortality is, Z = 1.88 with confidence interval ranged from 1.32 to 2.45. The annual instantaneous natural mortality at 29.5°C is, M = 1.64 and the estimated instantaneous fishing mortality is F = 0.24. 50% of G. decadactylus specimen were vulnerable to the Gill net “Soovi” at length L50 = 15.43 cm (Figure 9). The longevity calculated from to the growth coefficient (K) is Tmax = 3.75 years. The exploitation rate for G. decadactylus by the Gill net “Soovi” in Benin near shore waters is 0.13. This rate is clearly weak at the optimum rate (Eopt = 0.5) (Pauly and Munro, 1984). It could concluded that G. decadactylus was under exploited.
, where TL is total length and t is the fish age at a moment. The mortality parameters of G. decadactylus fishing by Gill net “Soovi” in the nearshore waters off Benin are presented in Figure 8. The annual instantaneous total mortality is, Z = 1.88 with confidence interval ranged from 1.32 to 2.45. The annual instantaneous natural mortality at 29.5°C is, M = 1.64 and the estimated instantaneous fishing mortality is F = 0.24. 50% of G. decadactylus specimen were vulnerable to the Gill net “Soovi” at length L50 = 15.43 cm (Figure 9). The longevity calculated from to the growth coefficient (K) is Tmax = 3.75 years. The exploitation rate for G. decadactylus by the Gill net “Soovi” in Benin near shore waters is 0.13. This rate is clearly weak at the optimum rate (Eopt = 0.5) (Pauly and Munro, 1984). It could concluded that G. decadactylus was under exploited.
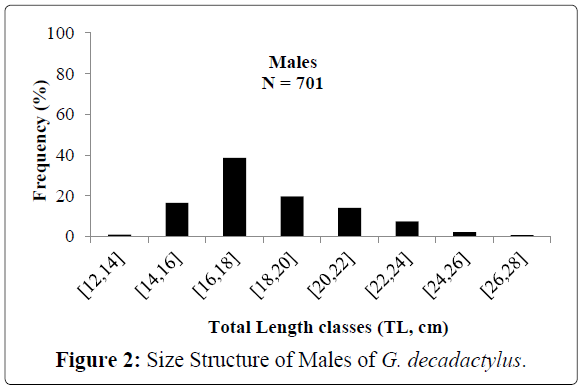
Figure 2: Size Structure of Males of G. decadactylus.
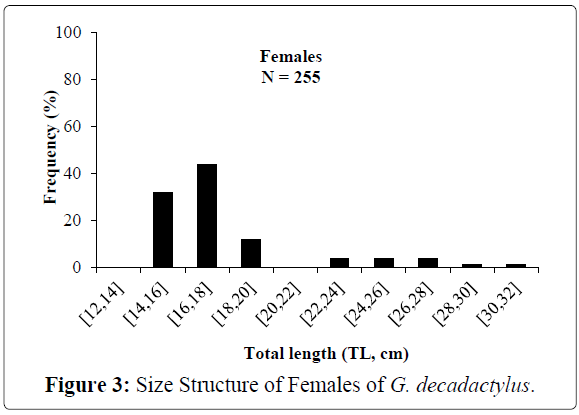
Figure 3: Size Structure of Females of G. decadactylus.
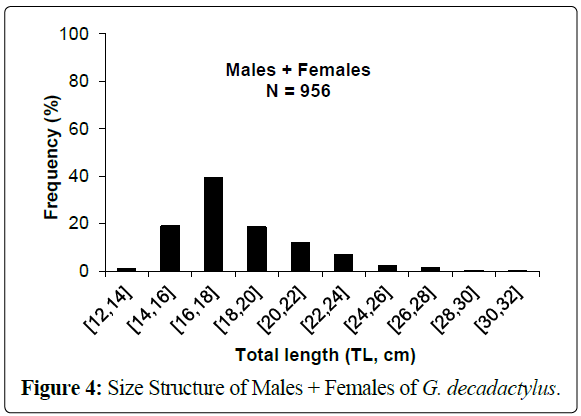
Figure 4: Size Structure of Males + Females of G. decadactylus.
| Length class |
12-14 |
14-16 |
16-18 |
18-20 |
20-22 |
22-24 |
24-26 |
26-28 |
28-30 |
30-32 |
TOTAL |
| M |
30 |
128 |
232 |
143 |
102 |
51 |
12 |
3 |
0 |
0 |
701 |
| F |
10 |
37 |
87 |
42 |
50 |
12 |
11 |
3 |
2 |
1 |
255 |
| Ratio M : F |
1:0.33 |
1:0.28 |
1:0.37 |
1:0.29 |
1:0.49 |
1:0.23 |
1:0.91 |
01:01 |
00:02 |
00:01 |
1:0.36 |
Table 1: Sex ratio in function of length class; M: Male; F: Female.
| Sexe |
N |
TL min (cm) |
TLmax (cm) |
W min (g) |
W max (g) |
Intercepta |
Slopeb |
r2 |
| M |
701 |
13.2 |
27.2 |
19.6 |
230.5 |
0.010 |
3.004 |
0.948 |
| F |
255 |
13.1 |
31.1 |
24.8 |
327.8 |
0.016 |
2.879 |
0.983 |
| M + F |
956 |
13.1 |
31.1 |
19.6 |
327.8 |
0.012 |
2.955 |
0.95 |
Table 2: Length-weight relationship parameters of males, females and combined sexes of G. decadactylus; M: Male; F: Female, N: Number of specimens; TLmin: Total Minimal Length; TLmax: Total Maximal Length; Wmin: Minimal Weight; Wmax: Maximal Weight; a: Intercept; b: Slope; r: Correlation Coefficient.
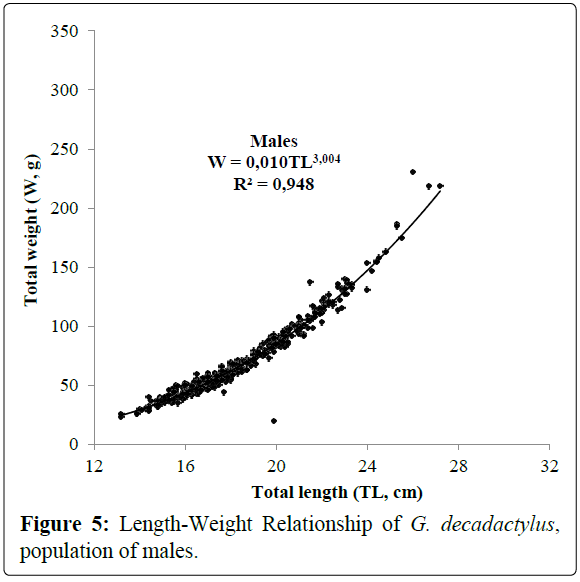
Figure 5: Length-Weight Relationship of G. decadactylus, population of males.
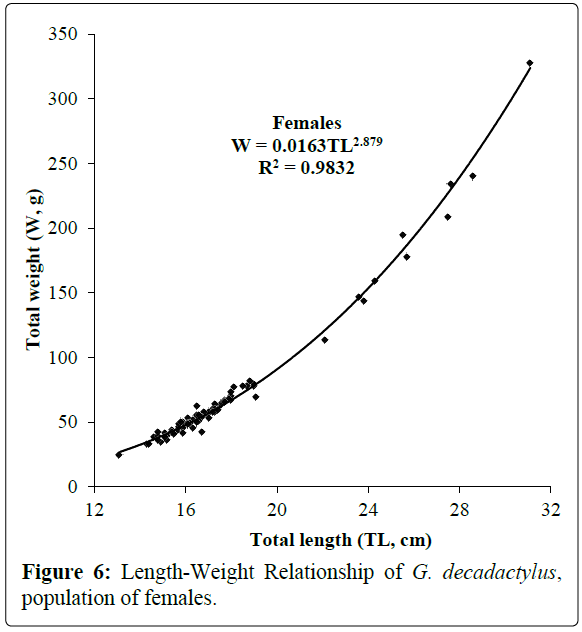
Figure 6: Length-Weight Relationship of G. decadactylus, population of females.
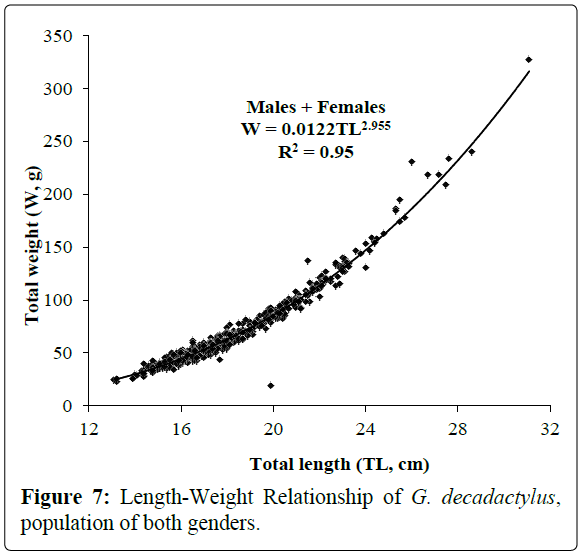
Figure 7: Length-Weight Relationship of G. decadactylus, population of both genders.
| Parameters |
Values |
| L ∞ (cm) |
26.25 |
| K (an-1) |
0.800 |
| t0 (ans) |
- 0.21 |
| j’ |
2.741 |
Table 3: Growth parameters of von Bertalanffy function output by FiSAT for G. decadactylus fishing by the Gill net “Soovi”
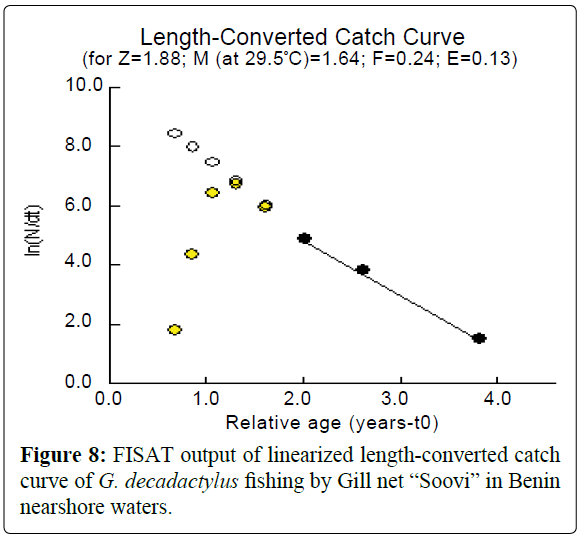
Figure 8: FISAT output of linearized length-converted catch curve of G. decadactylus fishing by Gill net “Soovi” in Benin nearshore waters.
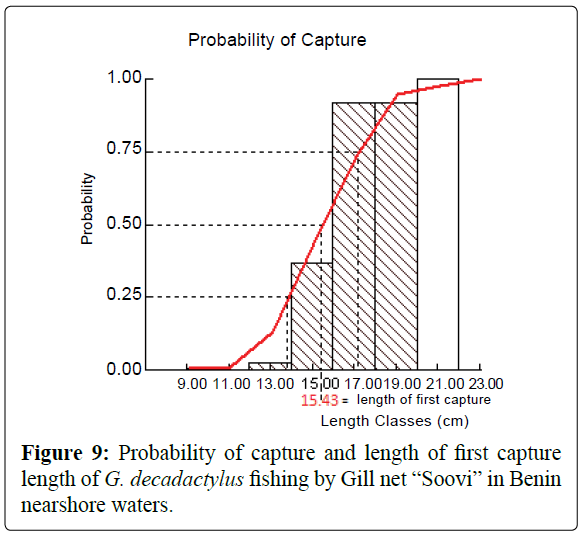
Figure 9: Probability of capture and length of first capture length of G. decadactylus fishing by Gill net “Soovi” in Benin nearshore waters.
Discussions
The population structure showed that small specimens are more vulnerable to the Gill net “Soovi. These results are similar to those obtained by Faggianelli (1984) in Togo. This author reported that the dominant lengths in catches are ranged from 15 cm to 20 cm. In the current study, the sex ratio is in favor of males. Ezekiel et al. (2013) in Nigeria found different results. In fact, Galeoides decadactylus is a species with a transitory hermaphroditism (Lopez, 1978), which counted in majority males with small length. This is in accordance with our results. The obtained value of “b” (b = 2.955) for the population in this study, showed a negative allometric growth. The fish’s length increase rapidly than its weight. Ezekiel et al. (2013) and Emmanuel et al. (2010) in Nigeria reported similar result. However, Fiogbé et al. (2003) in Benin and Konan et al. (2012) in Ivory Coast found an isometric growth for this species while N’Jock (1990) in Cameroon reported a positive allometric growth. These differences can be attributed to difference in sex, season, and time of sampling and environmental conditions. Moreover, environmental conditions had an effect on the length and the weight of fishes (Kundsen, 1962). Table 4 shows several values of length-weight relationship obtained on G. decadactylus in many studies. The value of asymptotic length in this study is different (lower) to those obtained by Samba (1974) in Guinea and Konan et al., (2012) in Ivory Coast. This difference can be explained by the selectivity of the Gill net “Soovi”, and by the diversity of determination methods of these parameters. The growth coefficient in this work is higher than those obtained in Guinea by Samba (1974) and in Ivory Coast by Konan et al. (2012). It could be concluded that the specimens of Benin stock grew faster than the Guinean’s. This difference was influenced by the upwelling and the target period. Indeed, the individual growth is conditioned by the reproduction characteristics of the species (temperature, available food), thus determined factors of individual growth. Growth performance index of G. decadactylus in the present study is lower than what found Konan et al. (2012) in Ivory Coast. The unfavorable environmental conditions and the weak food availability could be the major reasons of this difference. The estimation of instantaneous natural mortality in this work was higher than those obtained by Cavarivière and Thiam (1994) in Senegal, and Konan et al. (2012) in Ivory Coast. This difference could be attributed to the specimen’s sensibility to changes of natural factors and to the life in a weak productivity environment. This specie in Benin near shore waters was more available for predators. There is a strong correlation between natural mortality rate and longevity. Table 5 gives different values of longevity and natural mortality rates. It could be concluded that when the natural mortality is weak, the longevity is high. The exploitation rate of G. decadactylus’s stock with the Gill net “Soovi” is E = 0.13. This stock is under exploited with this gear. In addition, the determination of the MSY (Maximum Sustainable Yield) i.e. the quantity of fish of this species needed to be taken in the environment per year for a sustainable exploitation could be assessed.
| Authors |
Countries |
Length-Weight Relationships |
| Currentstudy |
Benin |
W = 0.012TL2.955 |
| Aggrey-Fynn et al., (2013) |
Ghana |
W = 0.011TL2.9893 |
| Ezekiel et al., (2013) |
Nigeria |
W = 0.2644SL1.9729 |
| Konan et al., (2012) |
Ivorycoast |
W = 0.007TL3.15 |
| Emmanuel et al., (2010) |
Nigeria |
W = -1.6948TL2.7962 |
| Fiogbé et al., (2003) |
Benin |
W = 0.0102TL3.02 |
| N’jock (1990) |
Cameroon |
W = 0.004TL3.15 |
Table 4: Length-Weight Relationship reported in other studies.
| Authors |
Tmax |
M |
| Current study |
3.75 |
1.64 |
| Konan et al., (2012) |
6.57 |
0.75 |
Table 5: Values of longevity Tmax and natural mortality rate.
Acknowledgments
We are grateful to all the fishermen of Cotonou Artisanal Fishing Port and to the authorities particularly Mr. EDO.
9876
References
- Adite, A. (2002) The mangrove fishes in the Benin estuarine system (Benin West Africa): diversity, degradation and management implications. Ocean docs-africa-african marine sciences-oceanography-fisheries
- nAggrey-Fynn, J., Fynn-Korsah, S., Appiah, N. (2013) Length-Weight Relationships and Food Preference of Two Coastal Marine Fishes, Galeoidesdecadactylus (Polynemidae) and Sphyraenasphyraena (Sphyraenidae) off Cape Coast, Ghana West African Journal of Applied Ecology, 21,87-96
- nAnato, C.B. (1999) Les Sparidae des côtesbéninoises: milieu de vie, pêche, présentation des espècesetbiologie de Dentexangolensis Poll et Maul, 1953, Thèse de Doctoratd’Etatès Sciences, Fac. Sci. 1060 Tunis, p: 277
- nCaveriviere, A.,etThiam, M. (1994)Taillesmoyennes et tauxd’exploitationapprochés des principalesespèces de poissonsdébarquées par les chalutiers glaciers entre 1973 et 1989. In L’évaluation des ressourcesexploitablespar la pêcheartisanalesénégalaise, Tome2.Edité par M. Barry-Gerard, T. Diouf, A. Fonteneau. ColloquesetSéminaire ORSTOM éds. Paris, pp: 365-384
- nChakroun-Marzouk, N.T., etKtari, M. H.(2003) Le Corb des côtestunisiennesSciaena umbra (Sciaenidae): Cycle sexuel, âge et croissance. Cybium, 27,211-225
- nChikou, A. (2006) Etude de la démographie et de l’exploitationhalieutique de six espèces de poissons-chats (teleostei, sliluriformes) dans le delta de l’Ouémé au Bénin ;Thèsedoct. sci., univ. Liège (belgique)
- nDirection des pêches., (2006)Statistiquessur la pêche maritime artisanale
- nDirection des pêches, (2012)Statistiquessur la pêche maritime artisanale
- nDomain, F. (1979) Note sur les périodes de reproduction de quelquesespècesdémersales du plateau continental sénégambien. Doc. Scient. Centre Rech. Océanogr. Dakar-Thiaroye, 68, 111-126
- nDomain, F. (1989) Rapport des campagnes de chalutage du N/O André Nizerydans les eaux de la Guinée de 1985 à 1988. Doc. Scient. CNSHB, Guinée. 81 pp
- nDulvyNK., Sadovy, Y., et Reynolds JD., (2003)Extinction vulnerability in marine Populations; fish and fisheries, 4, 25-64p
- nEdema, C.U., Osagiede, H. I. (2011)Morphological and trophic status of three polynemid Fishes from the warri river, Nigeria; Nigerian Journal of Agriculture, Food and Environment,7, 53-57.
- nEkouala, L., (2013) Le développement durable et le secteur des pêches et de l'aquaculture au Gabon: uneétude de la gestion durable des ressourceshalieutiques et de leurécosystèmedans les provinces de l’estuaire et de l’ogooué maritime; Thèse pour l’obtention du titre de docteur en géographieUniversité du Littoral côted’opaleécoledoctoralesesam (e.d n°73) laboratoiret.v.e.s (e.a n°4477) 408pp
- nEmmanuel, B.E., Gbesan K.,Osibona, A.O. (2010) "Morphology, Fecundity and diet of Galeoidesdecadactylus (Pisces: Polynemidae) (Bloch, 1975) off Nigerian coast," Nature and Science, 8, 15-23
- nEzekiel, M.O., Olusola, A.O., Edah, B., Udoezika, U.C., (2013) Fecundity, food and feeding habits and growth pattern of Galeoidesdecadactylus in Nigeria coastal waters; International Journal of Natural Sciences Research, 1, 1-13
- nFaggianelli, D. J., (1984) La pêcheartisanaledémersale au Togo, Centre ORSTOM de Lomé
- nFeltes, R.M., (1993) Parapolynemus, a new genus for the polynemid fish previously known as Polynemusverekeri. Copeia, 207-215
- nFiogbé, E.D., Sohou, Z., Gbaguidi, A., Hounkpe, C.,etDégbé, J-B. (2003) Les relations morphométriques des espèces de poissonscommerciaux du Bénin, ACP-EU Fisheries Research Report N°14
- nFischer, W., Bianchi, G.,Scott, W.B., (1981) FAO species identification sheets for fishery purposes.Eastern Central Atlantic, fishing areas 34, 111
- nGayanilo, F.C., Pauly, D. (1997)FAO ICLARM stock assessment tools (FiSAT): User’s Manual, FAO Computerized Information Series (Fisheries) (8), Rome, FAO, 262 p
- nGbaguidi, A., etFiogbe, E.D. (1999) Rapport national sur la pêche au Bénin 8pp
- nGréboval, D., (1999) Managing fishing capacity: selected papers on underlying concepts and issues. FAO fisheries technical paper n°386, Rome, FAO
- nKaiser, M.J., Colliem J-S., Hall, S.J., Jennings, S., Poiner I.R. (2002) Modification of marine habitats by trawling activities: prognosis and solutions; fish and fisheries, 3, 114-136
- nKébé, M., Anato, CB., et Gallène, J. (1997)Revue sectorielle de la pêcheartisanale au Bénin., Programme pour le DéveloppementIntégré des PêchesArtisanales en Afrique de l'Ouest (DIPA), 50p
- nKing, M. (1995) Fisheries Biology, Assessment and Management. Fishing News Books, Blackwell Science, Osney Mead, Oxford OX2 OEL, England, 341 pp
- nKonan, Kouassi, S., Diaby, M., Agnissan, Aka, J.P., Kone, A., etN’da. K. (2012) Croissanceetâge des poissonscapitaines: Polydactylusquadrifilis (Cuvier, 1829), Galeoidesdecadactylus (Bloch, 1795) et Pentanemusquinquarius (Linné, 1758) de la pêcherieartisanale maritime de Grand-Lahou (Côte d’Ivoire), Int. J. Biol. Chem. Sci, 6, 1112-1127
- nKonan, Kouassi, S., Kone, A., Akadje, C.M.A., Diaby, M., etN’Da, K. (2013) Ecologie des poissonscapitaines: Polydactylusquadrifilis (Cuvier, 1829), Galeoidesdecadactylus (Bloch, 1795) et Pentanemusquinquarius (Linné, 1758) de la pêcherieartisanale maritime de Grand- Lahou (Côte d’Ivoire), Tropicultura, 31, 187-193
- nKundsen, B., (1962)Growth and reproduction of house Mice at three different temperature Olkos, 13,1-14
- nLalèyè, P. (1995) Ecologiecomparée de deuxespèces de Chrysischthys, poissonssiluriformes (Claroteidae) du complexelagunaire lac Nokoué - lagune de Porto-Novo au Bénin.Thèse de doctorat, Université de Liège, Liège, 152pp
- nLe Lceuff, P., etIntes A.(1973)Note sur le régime alimentaire de quelquespoissonsdémersaux de côted’ivoire, Doc.Scient. Centre Rech. Océo", Abidjan 4, 17-44
- nLevêque, C., Paugy, D. (1999) Impacts des activitéshumaines. in les poissons des eauxcontinentalesafricaines. Diversité, écologie, utilisation par l’homme, Levêque C, Paugy D (eds). Institut de Recherche pour le Développement, Paris, 365-383
- nLonghurst, A.R. (1957)Food of the demersal fish of a West African estuary. J. Anim. Ecol, 26, 369-387
- nLopez, J. (1978)Ecologie, BiologieetDynamique de Galeoidesdecadactylus (Bloch, 1795) du plateau continental séné-gambien. Thèse de Doctorat de 3e cycle d'océanographie, Université de Bretagne Occidentale, Brest, France
- nMullon, C., Fréon, P.,etCury P. (2005)The dynamics of collapse in world fisheries, fish and fisheries, 6, 111-120
- nN'Jock, J.C. (1990) Les ressourcesdémersalescôtières de Cameroun :biologie et exploitation des principalesespècesichtyologiques. ThèseDoct.3ème cycle, Univ. Aix-Marseille 2, France.187 pp
- nPauly, D. (1979) Theory and management of tropical multispecies stocks: a review with emphasis on the Southeast Asian demersal sheries. J. Cons. CIEM Stud. Rev 1, 35
- nPauly, D. (1980) On the interrelations between natural mortality, growth parameters and mean environmental temperature in 175 sh stock. J. Cons. CIEM, 39, 175-192
- nPauly, D., Munro, J.L. (1984)Once more on the comparison of growth in sh and invertebrates. ICLARM Fishbyte, 2, 21
- nRicker, W.E.(1975)Computer and interpretation of biological statistics of sh population. Bull. Res. Board-Cam., 315-318
- nSamba, G. (1974) Contribution à l'étude de la biologie et de la dynamique d'un PolynemidaeOuestAfricainGaleoidesdecadactylus (Boch., 1795). Thèse de 3e cycle, Université de Bordeaux I, France. 114 pp
- nSidibé, A. (2010) Evaluation-Test surl’utilisation de la Liste Rouge de l’UICNcommeoutil de suivi des risques de perte de biodiversité : Application aux espèces de poissonsdémersauxcôtiersexploités en Afrique du Nord-Ouest; 66 pp
- nSossoukpe, E., Nunoo, F.K.E., Ofori-Danson, P.K., Fiogbe, E.D., Dankwa, H.R. (2013) Growth and mortality parameters of P. senegalensis and P. typus (Sciaenidae) in nearshore waters of Benin (West Africa) and their implications for management and conservation ; Fisheries Research 137,71-80
- nSossoukpe, E., Djidohokpin, G., Fiogbe,E D. (2016) Demographic parameters and exploitation rate of Sardinellamaderensis (Pisces: Lowe 1838) in the nearshore waters of Benin (West Africa) and their implication for management and conservation. International Journal Fisheries and Aquatic Studies4, 165-171
- nSparre, P., Venema, S.C. (1992) Introduction to tropical sh stock assessment. Part 1.Manual, FAO Fisheries Technical paper, 306. No 1, Review 1, FAO Rome 376 pp
- nVillanueva, M.C.S. (2004) Biodiversitéet relations trophiquesdansquelquesmilieuxestuariens et lagunaires de l’Afrique de l’Ouest: Adaptation aux pressionsenvironnementales. Thèse de doctorat, I.N.P. EcoleNationaleSupérieureAgronomique de Toulouse, France, 224 pp
- nVon Bertalanffy, L. (1938) A quantitative theory of organic growth. Human Bio10, 181-213








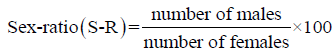

 , where TL is total length and t is the fish age at a moment. The mortality parameters of G. decadactylus fishing by Gill net “Soovi” in the nearshore waters off Benin are presented in Figure 8. The annual instantaneous total mortality is, Z = 1.88 with confidence interval ranged from 1.32 to 2.45. The annual instantaneous natural mortality at 29.5°C is, M = 1.64 and the estimated instantaneous fishing mortality is F = 0.24. 50% of G. decadactylus specimen were vulnerable to the Gill net “Soovi” at length L50 = 15.43 cm (Figure 9). The longevity calculated from to the growth coefficient (K) is Tmax = 3.75 years. The exploitation rate for G. decadactylus by the Gill net “Soovi” in Benin near shore waters is 0.13. This rate is clearly weak at the optimum rate (Eopt = 0.5) (Pauly and Munro, 1984). It could concluded that G. decadactylus was under exploited.
, where TL is total length and t is the fish age at a moment. The mortality parameters of G. decadactylus fishing by Gill net “Soovi” in the nearshore waters off Benin are presented in Figure 8. The annual instantaneous total mortality is, Z = 1.88 with confidence interval ranged from 1.32 to 2.45. The annual instantaneous natural mortality at 29.5°C is, M = 1.64 and the estimated instantaneous fishing mortality is F = 0.24. 50% of G. decadactylus specimen were vulnerable to the Gill net “Soovi” at length L50 = 15.43 cm (Figure 9). The longevity calculated from to the growth coefficient (K) is Tmax = 3.75 years. The exploitation rate for G. decadactylus by the Gill net “Soovi” in Benin near shore waters is 0.13. This rate is clearly weak at the optimum rate (Eopt = 0.5) (Pauly and Munro, 1984). It could concluded that G. decadactylus was under exploited.






