Key words
Sex reversal, Monosex, Male, Inversion, Methyltestosterone
Anahtar Kelimeler
Cinsiyet dönü?ümü, Monoseks, Erkek, ?nversiyon (de?i?me), Metiltestosteron
Introduction
Tilapia (Oreochromis niloticus) is one of the most important freshwater species cultured in many parts of the world. They consume low cost feed but grow fast, show tolerance to low water quality and tilapia meal is a good protein source for human consumption throughout the world as well as Thailand. Nile Tilapia was introduced from Japan to Thailand in 1965 as the King’s souvenir. The fish were propagated at the King’s palace and then distributed to Thai people the following year. In 2008, tilapia production of 217,200 tons equaled 41.6% of the total aquaculture production at the value of US$ 262.4 million (Anonymous, 2010). Tilapia is the most economic freshwater cultured species for domestic consumption and export.
As the male tilapias grow faster than females, culture of a single sex stopped the fish breeding. In addition, Beardmore et al. (2001) reported benefits of monosex male culture on high growth, preventing large energy diversion into ovary as well as reproductive behavior, reducing aggressive interaction and uniform size at harvest. Thus, culture of male tilapia is the most favored way for commercial scale farming (Mires, 1977; Guerrero, 1982). However, the male tilapia can be cultured from fry, produced by either manually sexing, hybridization or hormonal sex reversal (Mires, 1983). The hormonal sex reversal is the most reliable and widely used method in many countries. 17α-methyltestosterone (MT) is the most commercial synthetic androgen used for effective masculinization of more than 42 fish species (Devlin & Nagahama, 2002). In addition, the MT is also commercially used in monosex-male tilapia hatcheries worldwide including Thailand. In the commercial scales tilapia hatcheries of Thailand, fish farmers are able to produce male tilapias with a percentage of 86-100% (Bhujel, 1998). The method however, had to be applied and conducted in hapas suspended in an earthen pond in order to reduce labor costs on cleaning the cement tanks otherwise used. In addition, the sex reversal process used in earthen pond might cause MT contamination to workers and the surrounding environment.
The sex reversal by immersion technique had been developed and conducted in salmonids (Baker et.al., 1988; Piferrer & Donaldson, 1989). The use of MT at 200-400μg/L immersed new hatched larvae for 2h and then repeated with another 2h immersion the following week resulting in 82-100% males (Baker et.al., 1988). Similarly, Piferrer & Donaldson (1989) reported on a single immersion of 6 days post hatching Oncorhynchus kisutch in 400μg/L MT for 2h resulted in 73% males. In tilapia, Yang Yi (1992) immersed 21- 30 day post-hatched tilapia fry in 5mg/L MT for 3 days and produced 90% males. However Fitzpatrick et al., (1999) got 79.3% males by immersing the 13 days post-fertilization (dpf) tilapia larvae in 200μg/L methyldihydrotestosterone for 2h. Doses and duration of hormone immersing tilapia are vastly different between 200μg/L up to 5mg/L and 2h up to 3 days. Nevertheless, aging tilapia on a different basis using either on ‘dph’ or ‘dpf’, may affect labile or sensitivity period, thereby affecting the efficacy of the technique. Thus, we decided to use the dpf aging basis throughout the study, and the tilapia at either egg (2dpf) and late fry (14dpf) stages were selected for examination of different doses and durations of sex reversal by immersion technique.
Materials and Methods
Experimental fish
A total of 32,500 tilapia samples used in this study were supplied from the KhonKaen Inland Fisheries Research and Development Center. Twenty five concrete spawning tanks (5x10x0.6m) containing 50 females and 25 males in the center were used and the fish were fed with 25% protein pellet at 1-2% daily and egg were taken weekly. Each batch of egg or larvae was kept individually in a metal bowl. They were classified into age (dpf) according to their em-bryonic development. Pigmented eggs (2dpf) were selected from 13 females. The eggs were cleaned with 100ppm formalin for 2 min in order to remove any external parasites and washed with fresh water 2-3 times before being placed into a plastic tray for the experiment. Thirty five eggs were randomly selected and put into a plastic bot-tle incubator containing 1L fresh water with an air stone to circulate the eggs during incubation. The experiment was carried out in 27.5 ±1.2o C water temperature.
Hormonal preparation
Hormonal stock solutions were prepared by dissolving 15 and 30mg 17α-methyltestosterone (MT; Fluka Chemie, Buchs, Switzerland) in 15mL absolute ethanol. Then, the MT stock solu-tion concentrations were established at 1,000 and 2,000μg/mL, respectively.
Experiments
Factorial of 2 factors consisting 3 dose levels (MT: 0, 250 and 500 μg/L) and immersing dura-tions (either 6, 12, 24, 48, 72 and 96h or 6, 12, 24 and 48h) were designed in 2 age groups (2 and 14 dpf) with 4 replicates each. At the beginning of the experiment, 250μL of each MT stock was randomly added into the bottle incubators (1L) at 2dpf age. Thus, the MT concentrations in the bot-tles were 250 and 500 μg/L, respectively. For the control, only 250μL absolute ethanol was added to replace the MT stocks. The eggs (2dpf) were immersed for 6, 12, 24, 48, 72 and 96h. Later, the hormone solutions were drained off and thor-oughly washed with fresh water. They were fur-ther incubated in the same bottles until 8dpf (start feeding fry). They were counted (70 fry) and transferred into plastic containers containing 2L of water. The fry were fed with fish meal 5 times a day. The containers were cleaned and water was changed daily. At 14dpf, the untreated fry from the same egg batch of 2dpf immersion were treated with either 250 or 500 μg/L MT stocks in the plastic containers (70 fry/2L) while controls were added with absolute ethanol in the same amount as MT stocks. The fry were statically immersed in MT solution for 6, 12, 24 and 48h before being drained off, thoroughly washed and nursed in the same manner as the 2dpf immer-sion. They were nursed in the plastic containers for 3 weeks then transferred into aquaria supplied with 30L of bio-filtered and recycled water. The water volume was then slowly increased accord-ing to fish sizes and densities. They were nursed in the aquaria until at least 60dpf.
Sexing fish and data analysis
Numbers of the remaining fry at the end of the experiment were recorded to assess survival rate. Fifty fry (62-65 dpf ages) from each aquarium were sampled, dissected for gonads and individu-ally phenotypic sexed using Aceto-carmine Squash Method (Guerrero and Shelton, 1974) under a binocular microscope (x100). Arch sine transformation of the male proportion was used prior to Analysis of Variance to analyze the ef-fects of doses, immersing durations and interac-tion using SPSS version 11.5. Chi-square test was also used to detect sex ratio compared to the con-trol. The differences were considered statistically when the p-value was equal or less than 0.05.
Results and Discussion
MT was used in the present study in order to find out efficacy of MT immersion (250-500 μg/L) at different durations (6-96h). Both dosages of MT immersion significantly increased the percentage of male (P<0.05) while the im-mersion duration and interaction showed no ef-fect. There were no significantly differences on survival rates of the fry when immersed at either 2 or 14 dpf ages (79.0 ±10.1 - 97.7 ±0.4 % and 71.8 ±7.4 – 83.5 ±3.2%, respectively; Table 1 and 2). These indicated the fry dead were random and had no effect on particular sexes.
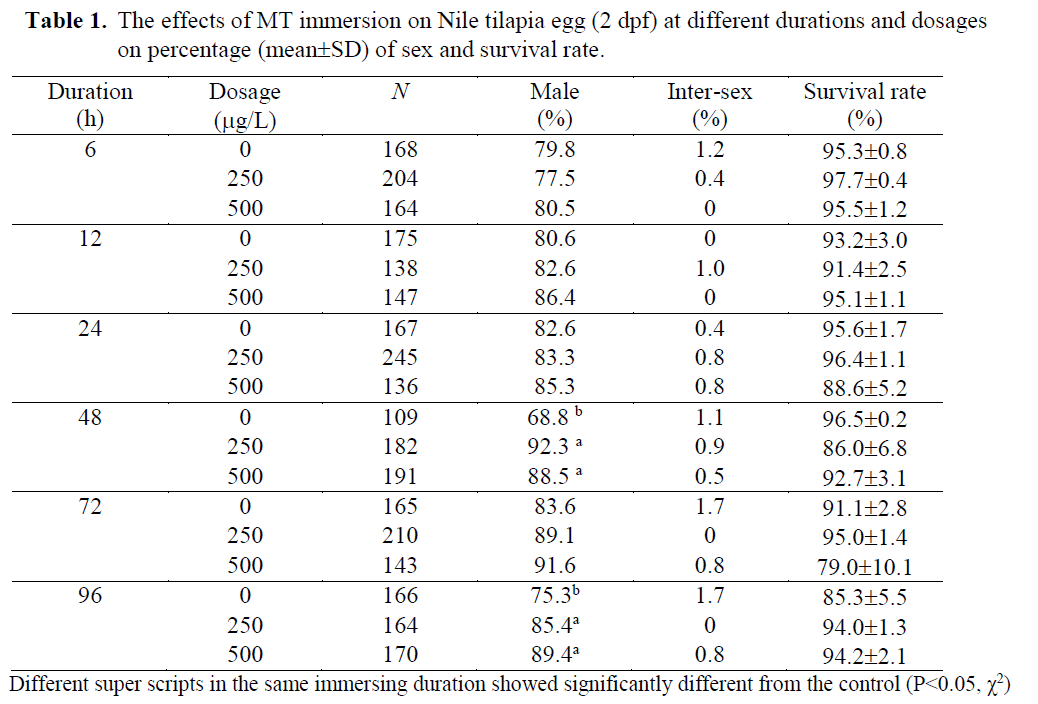
Table 1. The effects of MT immersion on Nile tilapia egg (2 dpf) at different durations and dosages on percentage (mean±SD) of sex and survival rate.
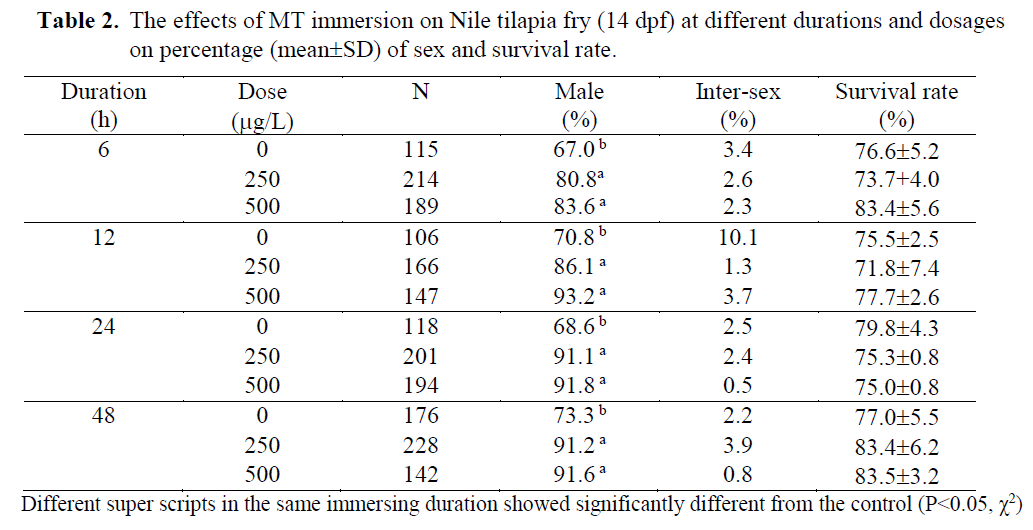
Table 2. The effects of MT immersion on Nile tilapia fry (14 dpf) at different durations and dosages on percentage (mean±SD) of sex and survival rate.
At egg stage (2dpf), the MT immersion (250 and 500μg/L) induced significantly (P<0.05) ma-les (85.4-92.3 MT immersions showed higher males (80.8-93.2 %) than those of the controls (67-73.3% males; Table 2). The results showed the possibility of the tilapia MT immersion stages at either eggs (2dpf) or late fry (14 dpf). In anot-her words, tilapia have a long labile period between 2 – 14 dpf before occurring gonadal dif-ferentiation and presenting some special cells, receptors or enzymes involved in steroid produc-tion (Devlin & Nagahama, 2002). The 14dpf age was correlated to the on- set of sex differentiation of Nile tilapia, which Srisakultiew & Rana (1991) indicated at the early age as 11-14 dpf at 27 ±2 °C. This finding is related to Baroiller et al.
(1996) who suggested the critical period of the fish to steroid sex reversal by oral application, must begin between 9-13dpf and last for 21 days (27 °C). Thus, the 14dpf was selected as the late fry immersing ages while the egg stage at 2dpf was used for comparing efficacy of MT sex re-versal in the present study, which resulted in sig-nificantly (P<0.05) higher males. The results in-dicated that both 2 and 14 dpf were sensitive ages (labile period or sex lability) for tilapia sex rever-sal. Similar to Haffray et al. (2009) who reported earlier and longer period of sex lability in brook trout, which needed to initiate several immersions during the week before hatching to 2 weeks after hatching and combined with oral MT treatment.
The single 48h MT immersion at either egg (2dpf) or fry stage (14 dpf) of the present study was found similarly 88.5-92.3 % (Table 1) or 91.2-91.6 % males (Table 2). These male percen-tages were similar to Yang Yi (1992) who im-mersed 21-30 dph tilapia fry for 3 days in the high MT dosage as 5mg/L and found 90% males. The MT dosage used by Yang Yi (1992) was about 10-20 times higher than the present study but resulted in similar male percentages. Thus, low MT dosage as 250 μg/L was found to be adequate for tilapia sex reversal by immersion technique at either egg or fry stages. Therefore, this technique could significantly reduce MT cost. In order to compare the MT immersion do-sages (250-500 μg/L) of the present study with oral MT dosage in standard protocol (60 mg/kg feed; Little et al., 1995), the immersion used only 0.42-0.83 % of the oral standard protocol while the oral used 120-240 times of the immersion. But the immersion resulted in a significantly inc-rease in the percentage of males (80.8-93.7 %) from the controls (67.0-83.6 %; Table 1-2). Thus, it was not necessary to use more or higher MT as Devlin & Nagahama (2002) summarized for ran-ges of MT dosage between 5-500 mg/kg feed due to a different application route.
The male percentages (80.8-93.7 %) by single immersion in the present study were higher than Pifferer & Donaldson (1993) who also did single 2h immersion at 6 dph salmonids (Oncorhynchus kisutch) in 400 μg/L MT and found 73 % males.
This may be due to longer immersing duration (6-96 h). For twice immersions at newly hatching salmonids and a week after for 2h in 200-400 μg/L MT found 82-100 % males (Baker et al., 1988). Also Haffray et al. (2009) reported that 3-4 immersions at a week before and a week after hatching, combined with orally administered MT had a higher effectiveness of sex reversal in brook trout. This indicated that the efficiency is increased in correlation with an increase in num-ber of immersions.
The immersion of the present study, however, had been carried out at a lower density as 35 eggs or fry/L when compared to brook trout (100 fry/L). Therefore, the efficacy of immersion at higher densities should be considered for com-mercial scale.
Conclusion
The MT immersion of tilapia egg (2dpf) for either 48 or 96 h induced significantly (P<0.05) males (85.4 –92.3%) as well as the fry immersion (14 dpf; 80 – 93% males). While the controls found 68.8 -75.3% and 67-73.3% males when immersed at egg and fry stage, respectively.
Acknowledgements
The authors would like to thank the Thai Re-search Funds, Office of the Prime Minister, Thai-land for funding (TRF; RDG4220018) the pro-ject. The authors would also like to thank Mr. Suchin Thongme, Ex-Director of Khon Kaen Inland Fisheries Research and Development Center for the supply of the tilapia eggs. Nevertheless, the authors would also like to send their special thanks to Prof. Dr. C. K. Lin for his valuable comments and feedback.
435
References
- Anonymous, (2010).Fisheries Statistics of Thai-land 2008.Information Center of Fisheries Department.Department of Fisheries, Mi-nistry of Agriculture and Cooperatives. Bangkok. 68p
- nBaker, I.J., Solar, I.I., Donaldson, E.M., (1988). Masculinization of chinook salmon (On-corhynchustshawycha) by immersion treat-ment using 17alpha-methyltestosterone aro-und the time of hatching, Aquaculture, 72(3-4): 359-369. doi: 10.1016/0044-8486(88)90224-4
- nBaroiller, J.F., Fostier, A., Cauty, C., Rognon, X., Jalabert, B., (1996). Effect of high rearing temperatures on the sex ratio of Progeny from sex reversed males of Oreochromisni-loticus. In: Pullin R.S.V., Lazard, J., Le-gendre, M., AmonKothias, J.B. and Pauly, D. (Eds.), The Third International Sympo-sium on Tilapia in Aquaculture, ICLARM Conf. Proc. Makati City, 41, pp. 246-256
- nBeardmore, L.A., Mair, G.C., Lewis, R.I., (2001).Monosex male production in finfish as exemplified by tilapia: applications, prob-lems and prospects, Aquaculture, 197: 283-301. doi: 10.1016/S0044-8486(01)00590-7
- nBhujel, R., (1998). Fry quality is the main factor behind the success of AIT’s joint venture ti-lapia hatchery, AASP Newsletter. Asian Ins-titute of Technology (AIT), 3(2):3-4
- nDevlin, R.H., Nagahama, Y., (2002). Sex deter-mination and sex differentiation in fish: an overview of genetic, physiological, and en-vironmental influences, Aquaculture, 208: 191-364.doi: 10.1016/S0044-8486(02)00057-1
- nFitzpatrick, M.S., Contreras-Sanchez, W.M., Miston, R.H., Lucero, M., Feist, G.W., Sch-reck, C.B., (1999).Steroid immersion for mazculinization of tilapia: Immersion for ti-lapia fry in MDHT. In: McElwee, K., Burke, D., Niles, M. and Egna., H. (Eds.), Sixteenth Annual Technical Report. Pond Dyna-mics/Aquaculture CRSP, Oregon State Uni-versity, Corvallis, Oregon. pp.73-74
- nGuerrero, R.D., (1982). Control of Tilapia repro-duction. In: Pullin, R. S. V. and Lowe-McConnell, R.H. (Eds.), The biology and culture of tilapias, ICLARM Conference Proceeding, Manila, pp. 309-316
- nGuerrero, R.D., Shelton, W.L., (1974). An aceto-carmine squash method of sexing juvenile fishes, Progressive Fish Culturist, 36: 56. doi: 10.1577/1548-8659(1974)36[56:AASMFS]2.0.CO;2
- nHaffray, P., Petit, V., Guiguen, Y., Quillet, E., Rault, P., Fostier, A. (2009). Successful pro-duction of monosex female brook trout Sal-velinusfontinalisusing gynogenetic sex re-versed males by a combination of methyltes-tosterone immersion and oral treatments, Aquaculture, 290: 47-52. doi: 10.1016/j.aquaculture.2009.01.029
- nLittle, D.C., Lin, C.K., Turner, W.A., (1995). Commercial scale tilapia fry production in Thailand, World Aquaculture, 26(4):20-24
- nMires, D., (1977). Theoretical and practical as-pects of the production of all-male tilapia hybrids, The Israeli Journal of Aquaculture- Bamidgeh, 29(3): 94-101
- nMires, D., (1983). Current techniques for the mass production of tilapia hybrids as practi-sed at EinHamifratz Fish Hatchery, The Is-raeli Journal of Aquaculture- Bamidgeh, 35(1): 3-7
- nPiferrer, F., Donaldson, E.M., (1989). Gonadal differentiation in coho salmon, Oncorhync-huskisutch, after a single treatment with androgen or estrogen at different stages du-ring ontogenesis, Aquaculture, 77: 251-262. doi: 10.1016/0044-8486(89)90207-X
- nPiferrer, F., Donaldson, E.M., (1993).Sex control in Pacific salmon.In: Muir, J.F. and Ro-berts, R. J. (Eds.), Recent Advances in Aqu-aculture.Institute of Aquaculture. Blackwell Science Publications, London, pp. 67-77
- nSrisakultiew, P., Rana, K.J., (1991). Effect of stocking density on sex differentiation of Oreochromisniloticus(L). In: Scott, A.P., Sumpter, J.P., Kime, D.E. and Rolfe, M.S.(Eds.), TheFourth International Symposium on the Reproductive Physiology of Fish. University of East Anglia, Norwich, U.K. FishSymp 91, Sheffield, p. 289
- nYang, Y., (1992).Investigation into the immer-sion of know-age Oreochromisniloticusju-veniles for hormonal sex reversal, 144p.Master’s thesis.Asian Institute of Techno-logy, Bangkok, Thailand.








