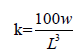Keywords
Cichlidae; Biometrical parameters; Growth pattern; Kainji Lake; Nigeria
Introduction
Despite the advent of techniques which directly examines biochemical or molecular genetic variation, conventional methods continues to have an important role in stock identification even to date (Swain & Foote, 1999). Morphological parameters and biometrical characteristics including morphometric measurement and meristic count have been used to identify fish stocks (Turan et al., 2004) and remain the simplest and most direct way among methods of species identification. The study of differences and variability in morphometric and meristic characters of fish stocks is important in phylogenetics and providing information for subsequent studies on the genetic improvement of stocks.
Environmental changes in the habitats of the fish due to human activities and continuous constructions along coastal lines as well as the pollution of the aquatic environment by fertilizers and pesticides, are expected to cause some morphological changes within species. Both morphometric and meristic characters respond to changes in environmental factors and these responses differ from species to species. Mohamed (1990), Goncalves et al. (1996), Froese & Pauly (1998) and Mwanja et al. (2011) had stated that morphological change and divergence within species are expected to take place when fishes are exposed to new developmental and evolutionary forces that determine their body forms. A change could take place, either through natural hybridization or the effect of the environmental factors that operate in early stages of development (Nei, 1987; Currens et al., 1989; Mohamed, 2010). The present study was, therefore, designed to compare the morphological characteristics of the cichlids species of Kainji lake by using a combination of both morphometric and meristic characters. The study also attempted to characterize the populations of these fishes in the lake and determine the morphological characteristics that contribute mostly to the variation of the cichlids in the lake, to our knowledge this is the first of such study aim at evaluating the morphological variation of fish in a dam constructed since 1968.
Materials and Methods
Kainji Lake, which is the largest man-made lake in Nigeria, was created in 1968 after the damming of River Niger for electricity generation by the National Electric Power Authority (NEPA). The Lake lies between Latitudes 9 0 50'and 100 55'N, and Longitudes 40 25'-40 45' E and between the borders of Sub-Saharan and Northern Guinea Savanna zones. It has a maximum length of 134 km, maximum width of 24.1 km, mean and maximum depth of 11 m and 60 m respectively, surface area of 1270 km2 , a volume of 13 × 109 m3, and catchment’s area of 1.6 x 106 km2 (Obot, 1989) (Figure 1).
Figure 1: Google map 2015 (Source).
Experimental Fish and Data Collections
A Total of 200 specimens of different species (50 for each species) of Cichlidae (Oreochromis niloticus, Tilapia zilli, Pelmatolapia mariae and Sarotherodon galilaeus) were obtained from the Kainji lake in February 2015 with sample collection done every day from all available landing site within the lake hydroelectric station. Biometrical parameters including morphometric measurement and meristic counts were determined as described by Samaradivakara et al. (2012). The morphometric variables included total length, standard length, dorsal fin length, anal fin length, pectoral fin length, pelvic fin length, pre-pelvic fin length, distance between occipital process, predorsal distance, eye diameter, body width, body depth, caudal penduncle depth, caudal fin length, head width, head length, vomerine length, vomerine width pectoral fin height, anal fin height and pre-orbital length. The meristic counts included anal fin ray, dorsal fin ray, caudal fin ray, pectoral fin ray, pelvic fin ray and dorsal fin spine. Body morphometric measurement such as total length, dorsal fin length, anal fin length, pectoral fin length, pelvic fin length, pre-pelvic fin length, pre-dorsal distance, body width, body depth, caudal penduncle depth, caudal fin length, pectoral fin height, anal fin height and dorsal fin height were expressed as percentages of standard length while head related morphometric parameters such as distance between occipital process, eye diameter, head width, vomerine length, vomerine width, snout length and pre-orbital length were expressed as percentages of head length.
The length-weight relationship was calculated using the equation given by LeCren (1951) and Ricker (1973) as follows
LogW=a+b logL
The function condition factor (K) for each species was calculated from the equation:
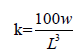
Where K=condition factor, L=Standard length (cm), W=Weight (g),
Statistical Analysis
To ensure that variations in this study were only attributed to body shape differences, and not to the relative sizes of the fish, size effects from the data set were eliminated, by standardizing the morphometric parameters using the allometric formula given by Elliott et al. (1995):
Madj=M (Ls/Lo) b;
Where M=original measurement, Madj=size-adjusted measurement, Lo=TL of the fish, Ls=overall mean of the TL for all specimens.
Parameter b was estimated for each character from the observed data as the slope of the regression of log M on log Lo, using all fish in all groups. However, it has been established that meristic characters are independent of size of fish hence should not change during growth (Strauss, 1985; Murta, 2000) therefore the raw data were analysed without transformation as described above. Statistical analyses in the present study included descriptive statistics using Minitab 14 as well as univariate analysis of variance using Genstat® discovery edition IV. Where significant differences occurred, Duncan’s least significant difference was used to separate the mean values of morphometric and meristic parameters. Morphometric and meristic data were subjected to discriminant function analysis (DFA) using Genstat® discovery edition IV.
Results
Morphological variations of cichlids shows significant differences in most morphometric parameters except in pectoral fin length, pelvic fin length, pre-dorsal distance, vomerine width and snout length (Table 1), all meristic count however were statistically different among the species (Table 2), S. galilaeus was observed to have higher values of morphometric parameters measured compared to other species. However, T. zilli had more meristic count than any other species under study.
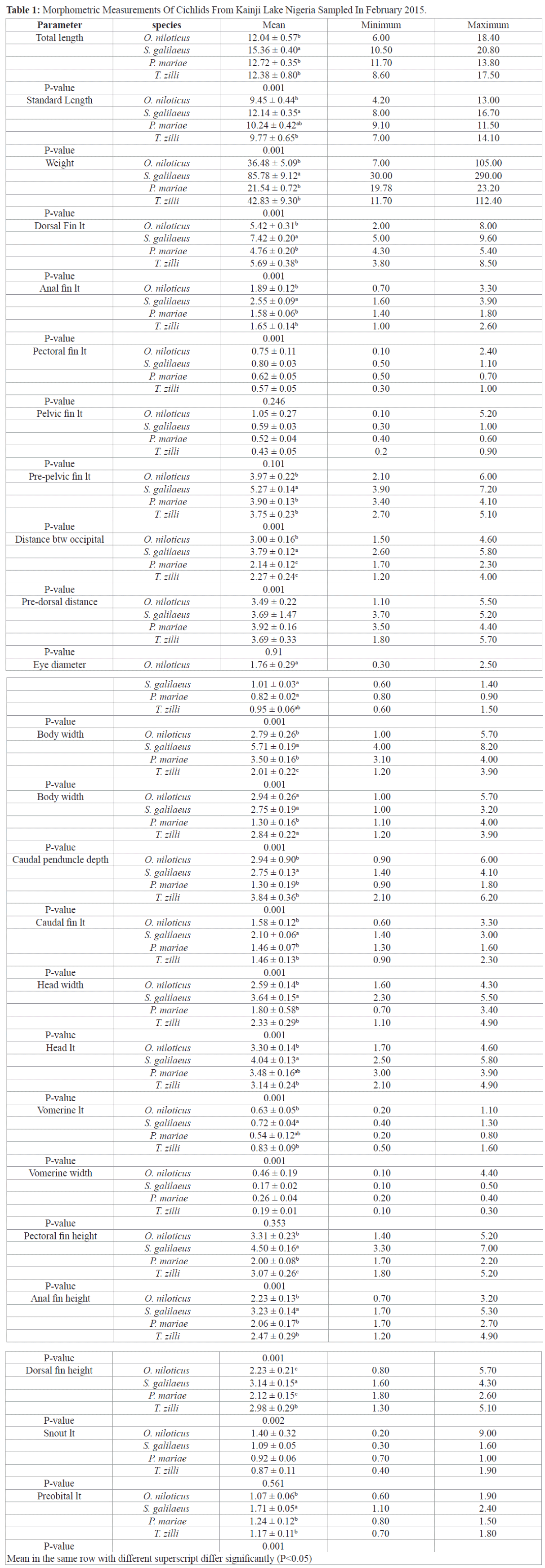
Table 1: Morphometric Measurements Of Cichlids From Kainji Lake Nigeria Sampled In February 2015.
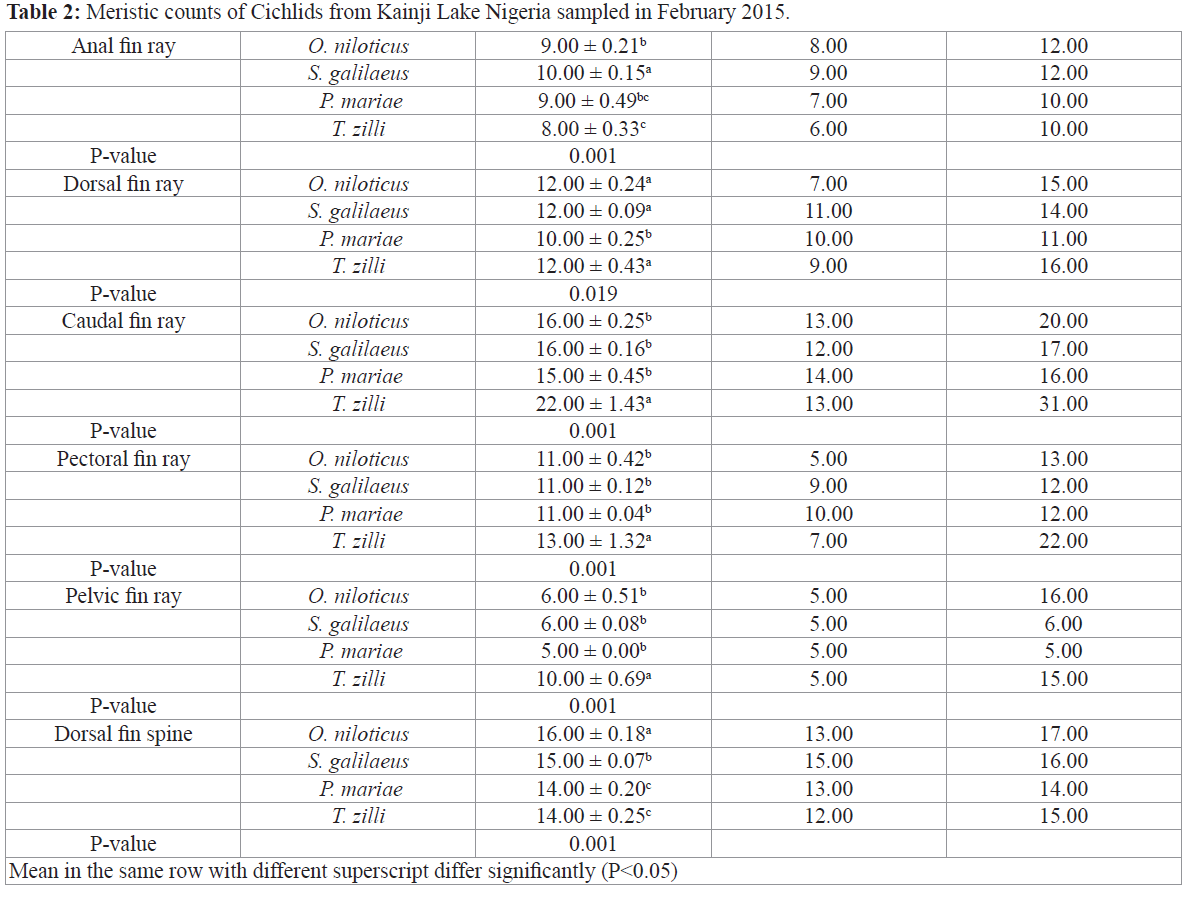
Table 2: Meristic counts of Cichlids from Kainji Lake Nigeria sampled in February 2015.
Expressing morphometric parameters as percentages of standard length (for body related parameters) and head length (for head related parameters) did not significantly change the trend of observation for most parameters as S. galilaeus still had higher percentages in ten out of fourteen parameter that were significantly different among the species (Tables 3 and 4).

Table 3: Morphometric measurements of cichlid from Lake Kainji sampled in February 2015 expressed as percentages of standard length.
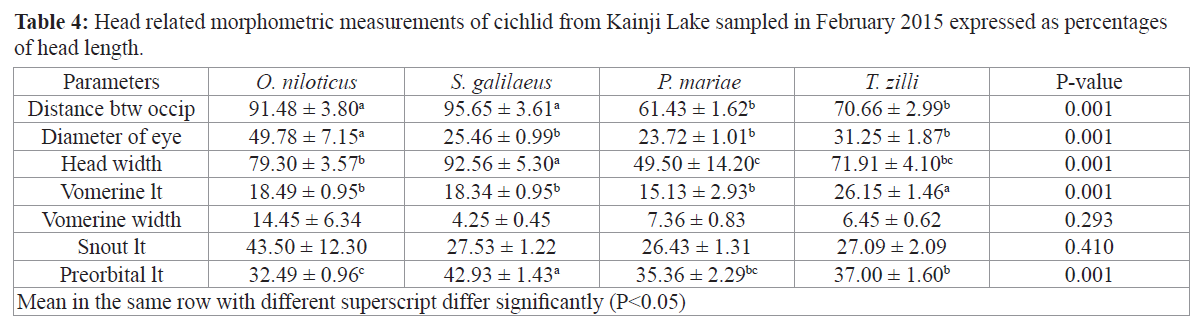
Table 4: Head related morphometric measurements of cichlid from Kainji Lake sampled in February 2015 expressed as percentages of head length.
Growth pattern of the different species reveals that O. niloticus, S. galilaeus and P. mariae had a negative allometric growth pattern (2.29, 2.47, and 0.72 respectively), while T. zilli had a positive allometric growth (3.26), condition factor however was higher in O. niloticus and S. galilaeus (4.16 and 4.27) and lower in P. mariae (2.06) (Table 5).
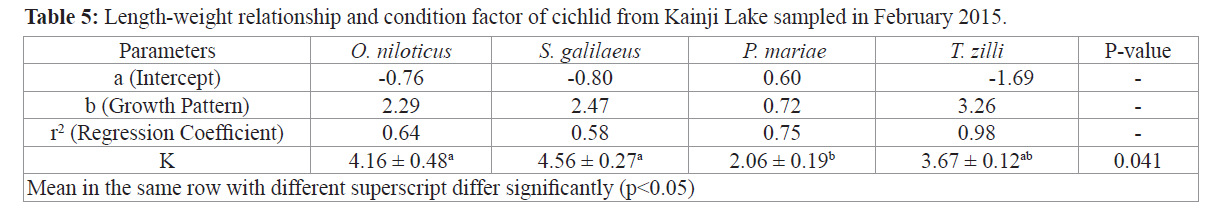
Table 5: Length-weight relationship and condition factor of cichlid from Kainji Lake sampled in February 2015.
Interspecific distance between the cichlids under study reveals the shortest distance between T. zilli and O. niloticus (14.70) while the longest distance was observed between T. zilli and S. galilaeus (52.40) (Table 6).
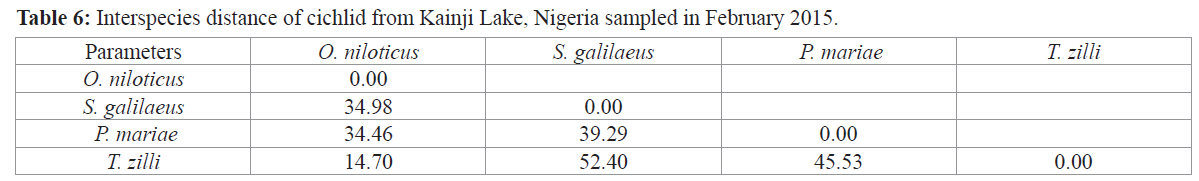
Table 6: Interspecies distance of cichlid from Kainji Lake, Nigeria sampled in February 2015.
Relationships of the morphometric measurement and meristic count analysis among cichlids from Kainji lake was considered according to the 1st and 2nd discriminate function (DF) (Figures 2 and 3 respectively). The 1st DF accounted for 42% and the 2nd DF accounted for 25% of among-group variability of the morphometric data, and together they explained 67% of total among-group variability. On the other hand, the 1st and 2nd DF of the meristic count analysis accounted for 47% and 27% respectively of the among-group variability, together they explained 74% of total among-group variability. According to the canonical discriminant function coefficients obtained for the morphometric data, the most influential variables for 1st DF were distance between occipital process, pre-dorsal distance, pectoral fin length, vomerine length, head length, head width, pre-pelvic distance while caudal fin ray, pelvic fin ray and pectoral fin ray constituted the most influential meristic variable for discrimination of the groups.
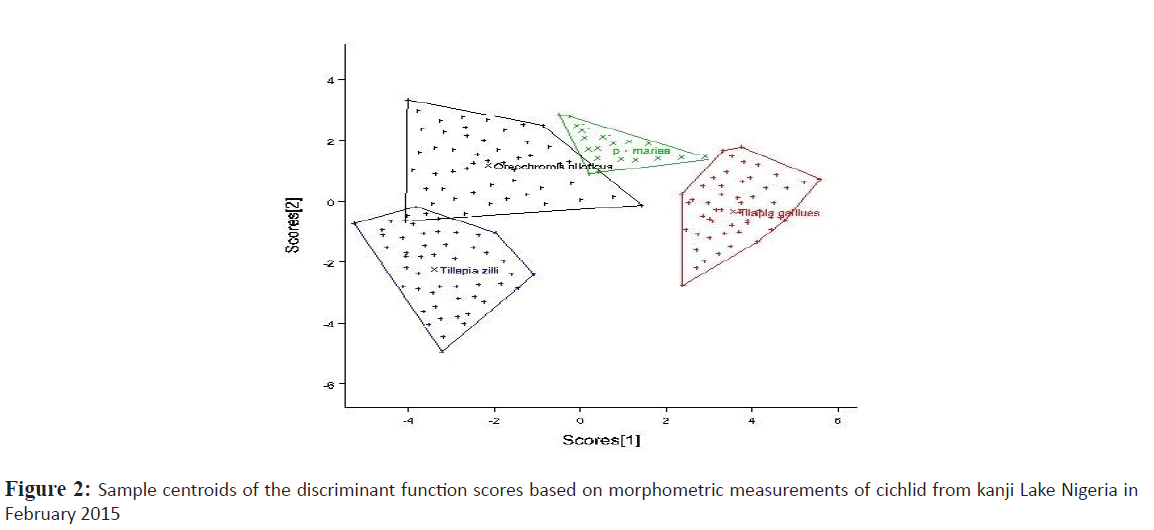
Figure 2: Sample centroids of the discriminant function scores based on morphometric measurements of cichlid from kanji Lake Nigeria in February 2015
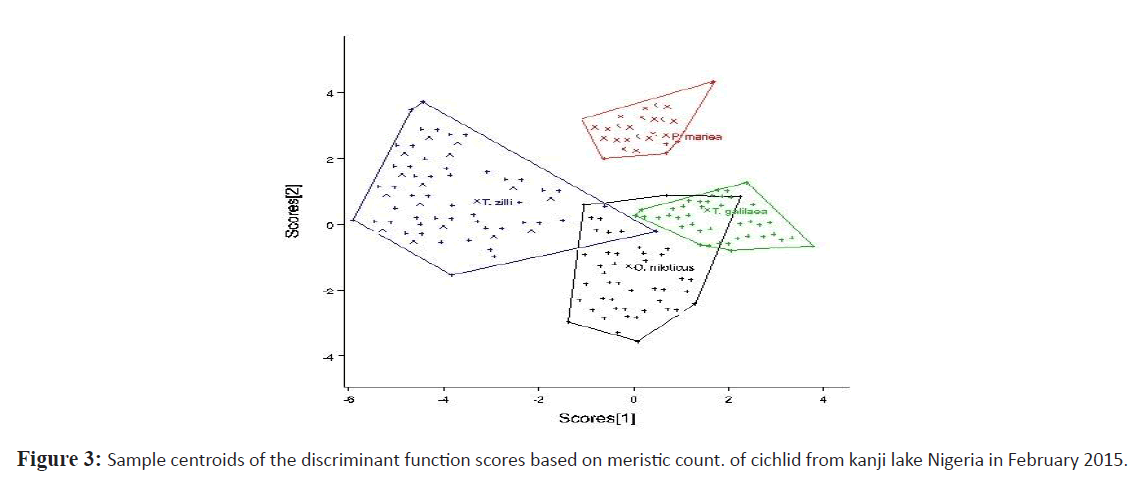
Figure 3: Sample centroids of the discriminant function scores based on meristic count. of cichlid from kanji lake Nigeria in February 2015.
Plots of canonical discriminant functions 1 of the morphometric measurements (Figure 2) clearly showed a complete separation between S. galilaeus and other species, hence a well separated and absolutely differentiated groupings along the first function, however there was noticeable overlap between O. niloticus and the other two species. Considering the 2nd DF, O. niloticus overlap broadly with P. mariae and S. galilaeus, however T. zilli only overlap broadly with S. galilaeus and slighthly with O. niloticus. For meristic counts, there were broad overlap between O. niloticus, S. galilaeus and P. mariae considering the first function. T. zilli clearly separate from other species but slightly overlap with O. niloticus. Second function however shows a significant overlap of T. zilli with all other species while overlap between O. niloticus, and S. galilaeus clearly separated from P. mariae.
Discussions
Fish has been said to demonstrate greater variances in morphological traits both within and between populations of species than any other vertebrates (Allendorf et al. 1987, Wimberger 1992). This study recorded significant difference in nine of fourteen body related morphometric parameters and six of eight head related parameters and in all meristic counts. Earlier studies by Beacham (1985), Beacham & Murray (1985), Beacham & Withler (1985), Beacham et al. (1988), Lund et al. (1989) and Kinnison et al. (1998) onSalmon has shown that morphometric parameters can be highly variable among and within conspecific populations, either correlating with geographical and habitat variation or having a genetic component, based on differences among groups in a common environment.
Allendorf and Phelps (1988), Swain et al. (1991) and Wimberger (1992) had highlighted environmental conditions such as food abundance and temperature as causes of fish high morphological plasticity, Solomon et al., (2015) had also suggested genetic variation caused by inbreeding, crossbreeding and other practices that can diluted gene pool as the major cause of differences in cultured and wild African catfish. However the marked differences of morphology in the present study may be linked to genetic differences of the species.
It has been reported by some fish biologists that ‘b’ values usually range from 2.0 to 4.0 for many fish species (LeCren 1951). According to the observation of the length-weight relationship of this study, all the species except P. mariea were within this range. Negative allometric growth implies the fish becomes more slender as it increase in weight while positive allometric growth implies the fish becomes relatively shorter or deeper-bodied as it increases in length (Riedel et al., 2007). This was evident in this study as T. zilli had shorter body width (20.40) while S. galilaeus, P. mariea and O. niloticus have significantly larger body width (46.99, 34.16 and 30.06 respectively). The value of “b” in GIFT and GIFU was reported to be 2.69 and 2.72 respectively by Shahririar Nazrul et al., (2011). Narejo et al. (1999) and Al-Baz and Grove (1995) also reported value of regression coefficient b in Tenualosa ilisha as 3.0246 for males and 3.0345 for females and 2.68 for males and 3.16 for females respectively. While Hile (1936) and Martin (1949) observed that the value of regression coefficient (b) usually lies between 2.5 and 4.0 in Leochthys artedi. However, differences in the ‘b’ value reported by the various authors is due to species variation, strain variation, stock variation, differences in environmental factors, sex variation etc. Higher condition factors were observed for S. galilaeus and O. niloticus while P. mariea had the least value. Differences in Condition factor can be due to different reasons which includes; stress, sex, season, availability of feeds, and other water quality parameters (Khallaf et al., 2003). Hence the availability and abundance of food at the time of sampling must have been the reason for the differences in the condition factor of the fish.
The values of relative condition factor in Shahririar Nazrul et al., (2011) experiment ranged from 0.897-1.06 for GIFT and 0.876-1.097 for GIFU and were lower than that recorded in the present study. For the discriminant analyses of the morphometric parameters, distance between occipital process, pre-dorsal distance, pectoral fin length, vomerine length, head length, head width, pre-pelvic distance contributed heavily to canonical discriminant function 1. While caudal fin ray, pelvic fin ray and pectoral fin ray constituted the most influential meristic variable for discrimination of the groups. Samaradivakara et al (2012) had earlier reported standard length, body height and pre-dorsal distance as major contributors to canonical discriminant function 1 in morphometric parameters of four Tilapia Populations in Selected Reservoirs of Sri Lanka. However, Haddon & Willis (1995) stated that Morphometrics of the head and body depth have been regarded as the most important characters for discrimination of angler fish (Lophius vormernus), Pacific herring (Clupea pallasi) and Orange roughy (Hoplostethus atlanticus) (Leslie & Grant, 1990; Schwegert, 1990; Haddon & Willis 1995) while Turan et al., (2005) reported HL as the only important parameter for discrimination of six population of African catfish in Turkey.
Eyo (2003) reported that among four Clarias species (Clarias ebriensis, C. albopunctatus, C. gariepinus and C. anguillaris), congeneric differences occurred in pectoral fin base length and frontal width, pelvic fin base length, Pectoral spine height, dorsal fin height, maxillary teeth band width, premaxillary teeth band depth, frontal, fontenelle length, internasal space, pelvic fin-anal fin space and prenasal barbell length, and in 6 residual characters namely Total Length, prepectoral length, pectoral fin base, length, dorsal fin base length, outer mandibular barbel space and eye diameter.
Specific differences among Distichodus species studied by Nwani and Ude, (2005) reveals that pelvic fin height, dorsal fin height, anal fin height, pectoral-pelvic fin space, pelvic anal fin space, head length and caudal peduncle depth were of significant taxonomic importance in discriminating all the studied Distichodus species. Nevertheless, in general, fishes demonstrate greater variance in morphological traits both within the same species or different species or between populations than other vertebrates and reflect differences in feeding environment, prey types, food availability or other features (Dunham et al., 1979; Allendorf, 1988; Thompson, 1991; Wimberger, 1992). It is also important to note that Among the principal morphological variables that aid in the discrimination this species and populations, some are related to feeding habits while the others are to swimming capacity and maintenance of the fish in the water column.
As mentioned before, Is there any difference in terms of feeding behavior or different depth layers of lake habitats of Cichlids species in the lake determined?
Overlapping variation in morphometric characters lead to great difficulty in identifying different stocks. Jerry and Cairns (1998) indicated that phenotype of an individual is a manifestation of its underlying genotype, as expressed in the local environment during development.
Consequently, individuals of different species that develop and mature in the environment or area would be expected to share a similar phenotype, as they are likely to experience common environmental and genetic influences (Chambers, 1993). Hence the noticeable overlap among different species for morphometric and meristic count. Vidalis et al. (1994) had argued that meristic characters may follow a predetermined variability at a very narrow range, and divergence of the meristic counts from a standard range could be fatal for the individual. Several authors have considered meristic characters less useful than the morphometric data (Misra & Carscadden, 1987) when comparing morphological variations, however, this study have shown that caudal fin ray, pelvic fin ray and pectoral fin ray constituted can be used to discrimination species of Tilapia. Generally the observable overlap among species despite genetic differences may have been as a result of similar species adaptations in response to the prevailing environmental conditions since the creation of the lake.
6893
References
- Al-Baz, A. F. and Grove, D. J.,(1995) Population biology of Sbour, Tenualosailisha (Hamilton-Buchanan) in Kuwait.Asian Fisheries Science., 8: 239-259
- nAllendorf FW, N Ryman, F Utter (1987) Genetics and fishery management: past, present and future in population genetics and fisheries management. Seattle, WA and London: Univ. of Washington Press, pp. 1-20
- nAllendorf FW, SR Phelps (1988). Loss of genetic variation in hatchery stock of cutthroat trout.Transactions. American Fisheries Society 109: 537-543
- nAllendorf, F.W.,(1988). Conservation biology of fishes.ConservationBiology 2:145-148
- nBeacham, T.D. & C.B. Murray (1985).Variation in length and body depth of pink salmon (Oncorhynchusgorbuscha) and chum salmon (O. keta) in southern British Columbia.Canadian Journal of Fisheries and Aquatic Sciences 42: 312-319
- nBeacham, T.D. & R.E. Withler, (1985). Heterozygosity and morphological variability of pink salmon (Oncorhynchusgorbuscha) from southern British Columbia and Puget Sound.CanadianJournalofGeneticsandCytology 27: 571-579
- nBeacham, T.D.,(1985). Meristic and morphometric variation in pink salmon (Oncorhynchusgorbuscha) in southern British Columbia and Puget Sound.CanadianJournalofZoology 63: 366-372
- nBeacham, T.D., R.E. Withler, C.B. Murray & L.W. Barner.(1988) Variation in body size, morphology, egg size, and biochemical genetics of pink salmon in British Columbia.Trans. Amer. Fish. Soc. 117: 109-126
- nChambers, R.C.,(1993). Phenotypic variability in fish populations and its representation in individual based models.Transactions of the American Fisheries Society, 122: 404-414
- nCurrens, K.P., Sharpe, C.S., Hjort, R., Schreck, C.B. and Li, H.W.,(1989) Effects of different regimes on the morphometrics of chinook salmon (Oncorhynchustshawytscha) and rainbow trout (O. mykiss). Copeia., 1989: 689-695
- nDunham, A.E., Smith, G.R. and Taylor, J.N.,(1979). Evidence for ecological character displacement in western American catostomic fishes.Evolution 33: 877-896
- nElliott NG, K Haskard, JA Koslow.,(1995). Morphometricanalysis of orange roughy (Hoplostethusatlanticus) off the continental slope of southern Australia.JournalofFishBiology 46: 202-220
- nEyo, J. E.,(2003). Congeneric Discrimination of Morphometric Characters among Members of the Pisces Genus: Clarias (Clariidae) in Anambra River, Nigeria. The Zoologist, 2(1): 1-17
- nFroese, R., Pauly, D.,(1998). Fishbase: concepts, design and data sources. Naga, 293 pp
- nGoncalves, J.M.S, Bentes, L., Lino, P., Ribeiro, J., Canario, A.V.M., Erzini, K.,(1996). Weight-length relationships for selected fish species of the smallscaledemersal fisheries of the south and south-west coast of Portugal.FisheriesResearch., 30: 253-256
- nHaddon, M. and Willis T.J.,(1995).Morphometric and Meristic comparison of orange roughy) Hoplosethusatlanticus: Trachichthyidae) from the Puysegur Bank and Lord Howe Rise, New Zealand and its implications for stock structure.MarineBiology 123:19-27
- nHile, R.,(1936). Age and growth of cisco.Leuchthysartedi Le suer in lake of north-eastern high lands.Bulletin, U. S. Bureau of Fisheries., 48: 211-317
- nJerry, D. and Cairns, S.,(1998): Morphological variation in the catadromous Australian bass, from seven geographically distinct riverine drainages. Journal of Fish Biology, 52:829-843
- nKhallaf, E., Galal, M., Athuman, M (2003).The biology of Oreochromisniloticus in a polluted canal.Ecotoxicology 12:405-416
- nKinnison, M., M. Unwin, N. Boustead & T. Quinn, 1998. Population-specific variation in body dimensions of adult Chinook salmon (Oncorhynchustshawytscha) from New Zealand and their source population, 90 years after introduction. CanadianJournalofFisheriesandAquaticScience 55: 554-563
- nLeCren, E. D.,(1951). The length weight relationship and seasonal cycle in gonad weight and condition in the perch (Percafluviatilis).Journal of Animal Ecology., 20: 201-219
- nLeslie, C.C. and Grant, W.S.,(1990). Lack of congruence between genetic and morphologicalstock structure of the Southern African anglerfish Lophiusvomerinus.SouthAfricanJournalofMarineScience 9:379-398
- nLund, R.A., L.P. Hansen & T. Jarvi.,(1989).Identification of reared and wild salmon by external morphology, size of fins and scale characteristics. NINA Forskningsrapp 1: 1-54
- nMartin, W.R.,(1949). The mechanics of environmental control of body form in fishes. Univesity of Toronto Study Biology., 56:1-91
- nMisra, R.K. and Carscadden, J.E.,(1987).A multivariate analysis of morphometrics to detect differences in populations of capelin (Mallotusvillosus).Journal du Conseil international pour l'Exploration de la Mer 43: 99-106
- nMohamed E.H.A.,(2010). Characterization of two Synodontis (Siluriformes: Mochokidae) catfish species in the White Nile and Lake Nubia. EnvironmentalBiologyofFish., 88: 17-23
- nMohammed, E.H.A.,(1990). Morphological and biochemical comparison of the catfishSynodontis from the White Nile and Lake Nubia. Ph.D. thesis, University of Khartoum,
- nMunasinghe, D.H.N and Thushari, G.G.N.,(2010). Analysis of morphological variation of four populations of Macrobraciumrosenbergii (Crustacea: Decapoda) in Sri Lanka. Cey.Journal of Science.(Biological Science.)39, 53-60
- nMurta, A.G.,(2000). Morphological variation of horse mackerel (Trachurustrachurus) in the Iberian and North African Atlantic: implications for stock identification. ICES JournalofMarineScience 57: 1240-1248
- nMwanja, M.T., Muwanika, V., Nyakaana, S., Masembe, C., Mbabazi, D., Justus Rutasire, J. and Mwanja, W.W.,(2011).Population morphological variation of the Nile perch (Latesniloticus, L. 1758), of East African Lakes and their associated waters.AfricanJournalofEnvironmentalScienceandTechnology.,5(11): 941-949
- nNarejo, N.T., Ali, S.S., Jafri, S.I.H. and Hussain, S. M.,(1999). A study on the age and growth of Palla, Tenualosailisha from the River Indus. Pakistan Journal of Zoology., 31(1): 25-29
- nNei, M.,(1987). Molecular Evolutionary Genetics . Columbia University Press, New York
- nNwani, C. D. and Ude, E. F.,(2005). Morphometric variations among three Distichodus species of Anambrariver, Nigeria. Animal Research International (2005) 2(3): 372-376
- nObot, EA : The macrophytic flora of the draw-down area of Lake Kainji, Nigeria. African Journal of Ecology 27: 173-177, 1989
- nRicker, W. E (1973). Linear Regression In Fishery Research. Journal of the Fisheries Research board of Canada, 30: 409-434
- nRiedel, R., Caskey, L.M., Hurlbert, S.H (2007). Lengthweight relations and growth rates of dominant fishes of the Salton Sea: implications for predation by fish-eating birds. Lake and Reservoir Management 23:528-535
- nSaliu J.K.,(2002) Size sex and seasonal dynamics in the dietary composition of Brycinus nurse from Asia reservoir, Ilorin Nigeria, ReviewofBiology 22: 105-109
- nSamaradivakara, S.P., Hirimuthugoda, N.Y. Gunawardana, R.H.A.N.M.Illeperuma, R.J.Fernandopulle, N.D., De Silva, A.D. and Alexander, P.A.B.D (2012). Morphological Variation of Four Tilapia Populations in SelectedReservoirs in Sri Lanka.Tropical Agricultural Research 23 2): 105-116
- nSchweigert, J.F.,(1990). Comparison of morphometric and meristic data against truss networks for describing Pacific herring stocks. In “Fish-Marking Techniques” (Ed.) by N.C. Parker, A.E. Giorgi, R.C. Heidinger, D.B. Jeter, E.D. Prince, G.A. Winana. American fisheries Society Sympsosium7 .American Fisheries Society, Bethesda, pp. 47-62.
- nShahriarNazrul K. M., Mamun A-A., Sarker B. S. and Tonny U. S.,(2011): Morphological variability of the 11th generation strain of nile tilapia, (Oreochromisniloticus) and traditional genetically improved farmed tilapia. Journal of Bangladesh Agricultural University 9(2): 345-349
- nSolomon S. G., Okomoda V. T., Ogbenyikwu A. I.,(2015). Intraspecific morphological variation between cultured and wild Clariasgariepinus (Burchell) (Clariidae, Siluriformes).Archives of Polish Fisheries.Vol 23 (1) Pp 53-61
- nStrauss, R.E.,(1985). Evolutionary allometry and variation in body form in the South American catfish genus Corydoras(Callichthydae). SystematicZoology 34: 381-396
- nSwain DP, BERidell, CB Murray.,(1991). Morphologicaldifferences between hatchery and wild populations of coho salmon (Oncorhynchuskisutch): environmental versus genetic origin. Canadian Journal of Fisheriesand Aquatic Science 48: 1783-1791
- nSwain, D.P. and Foote, C.J.,(1999). Stocks and chameleons the use of phenotypic variation in stock identification.Fisheries Research 43:113-128
- nThompson, J.D.,(1991). Phenotypic plasticity as a component of evolutionary change.Trendsin Ecology and Evolution 6:246-249
- nTuran C, D Erguden, F Turan, M Gurlek.,(2004a). Geneticand morphologic structure of Liza abu(Heckel, 1843) populations from the Rivers Orontes, Euphrates and Tigris.Turkish Journal of Veterinary and Animal Science 28: 729-734
- nTuran C, M Oral, B Ozturk, E Duzgunes.,(2005). Morphometric and meristic variation between stocks of bluefish (Pomatomussaltatrix) in the Black, Marmara, Aegean and northeastern Mediterranean Seas. FisheriesResearch 79: 139-147
- nVidalis, K., Markakis, G. and Tsimenides, N.,(1994). Discrimination between populations of picarel (SpicarasmarisL., 1758) in the Aegean Sea, using multivariate analysis of phonetic characters.FisheriesResearch30, 191-197
- nWimberger, P.H.,(1992). Plasticity of fish body shape, the effects of diet, development, family and age in two species of Geophagus(Pisces: Cichlidae). BiologicalJournalofLinneanSociety 45, 197-218.