Key words
Astronotus ocellatus, Gussevia asota, Parasites of fish, Peruvian Amazon
Introduction
In fish farming the intensive exploitation al-lows the handling of high densities of organisms per unit area. Indeed, this type of management frequently leads to break the balance between pathogen and host, consequently resulting in the emergence of infectious and parasitic diseases that cause various problems ranging from slow up growth, reduced fertility rates, until the ap-pearance of severe epidemics resulting in high mortality (Thatcher 1991; Scholz 1999; Cable et al., 2002).
Cichlids have a wide geographical distribu-tion. Currently there are 1,533 known species, with 320 reported for South America (Kullander 1988). These species inhabit a wide variety of aquatic ecosystems. Moreover, the fish represent high economic importance, given that they are marketed for human nutrition with a promissing potential for intensive and extensive aquaculture (Kullander and Ferreira 2006; Araujo et al., 2009).
The oscar Astronotus ocellatus (Agassiz, 1831) can reach up to 45 cm in length and 1.6 kg of total weight (Fracalossi et al., 1998) and Oscar is a much appreciated species for its meat, which has a firm consistency and lacks intramuscular bones with great acceptance on the Amazonian market being regarded as a food fish of the high-est quality. The A. ocellatus is normally found in Amazon floodplain areas, and is characterized as a hypoxia tolerant species (Almeida-val et al., 1995; Almeida et al., 2000). Recent experiments carried out in laboratory have shown that the adult animals tolerate 6 h anoxia at 28°C by re-ducing their standard metabolic rates (Muusze et al., 1998). Due to its zootechnical characteristics, the A. ocellatus is considered a species with great potential for management in controlled environ-ments aiming human nutrition and ornamental purposes. However, to allow the breeding to be-come entirely feasible, it turns out the necessity to solve the problem of diseases and parasites up-surge affecting this species in controlled envi-ronments, as a consequence of intensive farming under inadequate management (Varella and Mal-ta 1995).
Therefore, with the gradual increase of inten-sive and semi-intensive fish farming in the Peru-vian Amazon, there is a need for constant moni-toring of the fish for the diagnosis and timely control of infestations by monogeneans. In this sense, the present study aims to evaluate the monogenean infestation in A. ocellatus bred in a fish farm in the Peruvian Amazon.
Material and Methods
Between July and August 2011, which corre-sponds to the relative dry season, 50 individuals of the species A. ocellatus were collected with drag nets, from a semi-intensive fish farm, locat-ed in the northeast of Loreto (Peru), between lati-tudes 3° 48' 48.9'' N and 073° 19' 18.2'' W, with average annual temperature of 26.3°C and rela-tive humidity of 85% at 328 mean sea level.
Fish were fed twice daily with extruded diet containing 25% crude protein and 2.6 Mcal/kg of digestible energy and feeding rate of 5% of the biomass of the pond. The sampled fish presented length of 12.60 ±0.10 cm and weight of 50.08 ±0.86 g. Having identified the parasite infesta-tion, the fish were transferred to concrete tanks covered with tiles to undergo long-term baths containing 0.5 to 2.0 ppm of potassium perman-ganate and 1% formalin during one hour. In the absence of improvement, we sacrificed and burned all the fish from the respective pond.
Using a stereoscope we examined the body surface, fins, nostrils, mouth, opercula and gills, looking for possible injuries and excess of mucus production. By means of a scalpel, we also per-formed scraping of the skin, fins and gills to ob-serve possible attached parasites.
For examination of the gills, the samples were separated and placed in glass containers with a 1:4,000 formalin solution. After one hour, the gills were stirred in the liquid and then removed from the container. Helminths were allowed to settle on the bottom and were subsequently col-lected with the aid of a small probe and a dissect-ing microscope (Nikon SM-30). The identifica-tion of the parasites was based on the methodolo-gy of Kritsky et al. (1989), Thatcher (2006) and Abdallah et al., (2008).
To study the monogeneans, permanent slides were prepared with total parasites assembly ac-cording to Thatcher (1991). For the study of scle-rotized structures, parasites were fixed in a solu-tion of ammonium picrate glycerine (GAP) and mounted in Canada balsam. Some specimens were mounted unstained in Gray and Wess' me-dium. To visualize internal structures, parasites were fixed in hot formaldehyde solution (4%) for staining with Gomori's trichrome. The parasitic indexes calculated for assessing the level of in-festation of parasites in the fish were prevalence, mean intensity and mean abundance (Bush et al., 1997).
Results and Discussion
The necropsy of juveniles from A. ocellatus bred in controlled environments in the Peruvian Amazon evidenced the infestation by the mono-genean G. asota in the gill filaments of the fish.
Indeed, the totality of the examined fish showed a high parasitic infestation by G. asota. The mean intensity was equal to the mean abun-dance, provided that the number of parasitized fish was the same as those examined (Table 1).
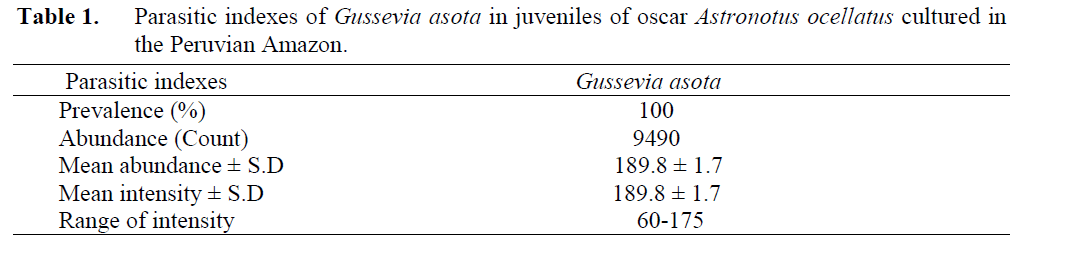
Table 1: Parasitic indexes of Gussevia asota in juveniles of oscar Astronotus ocellatus cultured in the Peruvian Amazon.
Several studies report the parasitism of neo-tropical cichlids by monogeneans belonging to the genus Gussevia Kohn and Paperma 1964 (Kritsky et al., 1989; Vidal-Martinez et al., 2001; Mendoza-Franco et al., 2010; Yamada et al., 2011; Mathews et al., 2013a). For south America thirteen species of Gussevia have been described for eight species of cichlids (Kritsky et al., 1986), evidencing a high specificity of the genus Gus-sevia in parasitizing cichlids.
In the Central and Peruvian Amazon several species of monogeneans of the genus Gussevia have been reported parasitizing cichlid of eco-nomic importance for human nutrition and orna-mental purposes (Kritsky et al., 1989; Mendoza-Franco et al., 2010; Azevedo et al., 2010). How-ever, little is known about the parasitic Infections of farmed A. ocellatus , because these studies have been carried out, in general, in wild fish. In our study we report for the first time the parasit-ism by the G. asota in juveniles of A. ocellatus bred in controlled environments in the Peruvian Amazon.
In the study described herein, the juveniles of A. ocellatus presented high levels of parasitism by the monogenean G. asota. Parasites that have a direct life cycle, such as monogeneans, are more frequently found in lentic environments. Moreover, this type of environment favors the transmission of these parasites (Flores-Crespo et al., 2003; Azevedo et al., 2007), which justifies the fact that the fish had elevated parasite infec-tion, since the same are confined to their culture in earthen ponds where water circulation is al-most negligible or nonexistent.
According to Buschmann (2001) and Mariano et al. (2010), intensive fish farming generates a large accumulation of organic matter on the pond bottom produced from the excreta, dead matter and the fraction of uneaten food. This organic matter produces hypoxia and anoxia that creates an unbalance in the homeostasis of the fish, even-tually leading to the increase of the oxidative stress of biomolecules, promoting thus various physiological and biochemical alterations, caus-ing cell impairment and death (Sherry 2003; Van der Oost et al., 2003). Therefore, these adverse effects of poor water quality reduce the self re-sistance of the fish, which turns out as a favora-ble condition to the parasite proliferation. The A. ocellatus is normally found in Amazon floodplain and is characterized as a hypoxia tolerant species (Almeida-Val et al., 1995). However, while adult animals are found in hypoxic waters, juveniles of this species are active and may be easily found in superficial water body layers, where oxygen availability is higher, suggesting a reduced capac-ity to tolerate hypoxia among juveniles. This fact may justify the high parasitic infestation by G. asota in A. ocellatus juveniles from fish farming.
The results described herein are in accordance with Kritsky et al. (1989), Azevedo et al., (2010), and Kim et al. (2002), who found the monogene-an G. asota parasitizing gills of A. ocellatus and, being common the setting of this kind of mo-nogenoide parasite in this organ (Kritsky et al., 1989). Indeed, several studies report the parasit-ism of G. disparoides in gills of H. severus, C. amazonarum and C. ocellaris and all these fish species are currently being raised in confined en-vironments in the Peruvian Amazon.
Furthermore, in a study with A. ocellatus cap-tured from the wild, Abdallah et al. (2008) found 62.8% of prevalence and mean intensity of 17.6 monogeneans of G. asota. However, the results differ from our study where we found a preva-lence of 100% and mean intensity of 189.8 para-sites of G. asota. A possible reason for the low levels of infestation reported by Abdallah et al. (2008) may be fact that the fish originate from nature. Nevertheless, Kritsky et al. (1989) found 100% prevalence of G. asota in A. ocellatus, alt-hough without informing other parasitic indexes.
Parasites of the genus Gussevia are considered specific for cichlids and therefore may show low susceptibility when present in favorable breeding conditions. Kritsky et al. (1989) mentioned that G. asota apparently can cause the death of its host, citing the case of an aquarium in Idaho, USA, as an example. This is the first report of G. asota parasitizing A. ocellatus in fish farming in the Peruvian Amazon. The results of this study and studies addressing various aspects of parasite in other species bred in the same region (Mathews et al., 2007; Dinis et al., 2007; Mathews et al., 2011; Mathews et al., 2013b; Mathews et al., 2013c) confirm the necessity of constant monitoring of fish, seeking the diagnosis and timely control of infestations by monogene-ans, in order to reduce fish mortality.
Conclusion
This is the first report of infestation high by G. asota in A. ocellatus cultured from the Peruvi-an Amazon. G. asota infection probably contrib-uted to the mortality of the captive cichlids.
Acknowledgments
The authors thank Dr. Omar Mertins for re-viewing this manuscript.
400
References
- Abdallah, V.D., Azevedo, R.K., Luque, J.L., (2008). Notes of the morphology on the two species of GusseviaKonh e Paperna (Monogena: Dactilmogyridae) parasitic on Astronotusocellatus(Agassiz) (Perciformes: Cichlidae) from Brazil, Pan-American Aquatic Sciencies, 3: 101-104
- nAlmeida-Val, V.M.F., Farias, I.P., Silva, M.N.P., Duncan, W.P., Val, A.L., (1995). Biochemical adjustments to hypoxia in Amazon Cichlids, Brazilian Journal of Medical and Biological Research, 28: 1257-1263
- nAlmeida, V.M.F., Val, A.L., Duncan, W.P., Souza, F.C.A., Paula-Silva, M.N., Land, S., (2000). Scaling effects on hypoxia tolerance in the Amazon fish Astronotusocellatus(Percifor-mes, Cichlidae): Contribution of tissue enzyme levels, Comparative Biochemical and Physiology, 125: 219-126. doi: 10.1016/S0305-0491(99)00172-8n
- nAraujo, C.S.O., Barros, M.C., Gomes, A.L.S., Varella, A.M.B., De Moraes, G.V., Da Silva, N.P., Da Costa E.F., Andrade S.M.S., (2009). Parasitas de populaçõesnaturais e artificiais de tucunaré (Cichlaspp.), RevistaBrasilera de ParasitologiaVeterinária, 18(1): 34-38. doi: 10.4322/rbpv.01801006 n
- nAzevedo, R.K., Abdallah, V.D., Luque, J.L., (2007). Ecologia da comunidade de metazoáriosparasitos do ApaiaríAstronotusocellatus(COPE, 1872) (Perciformes: Cichlidae) do rioGuandu, Estado de Rio de Janeiro, Brasil, RevistaBrasilera de ParasitologiaVeterinária, 16: 15-20
- nAzevedo, R.K., Abdallah, V.D., Luque, J.L., (2010). Acanthocephala, Annelida, Arthropoda, Myxozoa, Nematoda and Platyhelminthes parasites of fishes from the Guandu river, Rio de Janeiro, Brazil, Journal of Species Lists and Distribution, 6(4): 659-667
- nBush, A.O., Lafferty, K.D., Lots, J.M., Shostak, W., (1997). Parasitology meets ecology on its own terms: Margolis et al. revisited, Journal of Parasitology, 83: 575-583.doi: 10.2307/3284227n
- nBuschmann, A.H., (2001). Impactoambiental de la acuicultura. El estado de la investigación en Chile y el Mundo. Registro de ProblemasPúblicos N°4. TerramPublicaciones. Santiago
- nCable, L., Tinsley, R.C., Harris, P.D., (2002). Survival and development of Gyrodactylus. Gasteriostei (Monogenea: Gyrodactylidae), Parasitology, 124: 53-68. doi: 10.1017/S0031182001008861 n
- nDinis, V.N., Mathews, D.P., Chu-Koo, F.W., Tello, S.M., Ismiño, O.R., (2007). Fauna parasitaria de juveniles de arahuana, Osteoglossumbicirrhosum(Vandelli, 1829) cultivado en el Centro de Investigaciones de Quistocoha, Loreto, Perú. Folia Amazonica, 16(1): 29-33
- nFlores-Crespo, J., Flores, R.C., (2003). Monogeneos, parásitos de peces en México: Estudiorecapitulaivo, TécnicaPecuaria México, 41(2): 175-192
- nFracalossi, D.M., Allen, M.E., Nicholsdagger, D.K., Oftedal O.T., (1998). Astronotusocellatus, Have a Dietary Requirement for Vitamin C, The Journal of Nutrition, 128: 1745-1751
- nKim, J.H., Hayward, C.J., Seong, J.J., Gang-Joon, H., (2002). Parasitic infections in live freshwater tropical fishes imported to Korea, Diseases of Aquatic Organisms, 52: 169-173. doi: 10.3354/dao052169 n
- nKullander, S.O., (1988). Cichlidema-SydamerikasBrokiga. Fauna Och Flora, 41: 56-167
- nKullander, S.O., Ferreira, E.J.G., (2006). A review of the South American cichlid genus Cichla, with descriptions of nine new species (Teleostei: Cichlidae). Ichthyological Exploration Freshwaters, 17(4): 289-398,n
- nKritsky, D.C., Thatcher, V.E., Boeger, W.A., (1986). NeotropicalMonogenea. 8. Revision of Urocleidoides(Dactylogyridae, Ancyrocephalinae). Proceedings of the Helminthological Society Washington, 53(1): 1–37
- nKritsky, D.C., Thatcher, V.E., Boeger, W.A., (1989). NeotropicalMonogenoidea 15.Dactylogyrids from the gills of Brazilian Cichlidae with proposal of Sciadicleithrumgen. n. (Dactylogyridae). Proceedings of the Helminthological Society Washington, 56(2): 128-140
- nMariano, M., Huaman, P., Mayta, E., Chanco, M., (2010). Pollution produced by intensive fish farming in Andean lagoons, Junín, Peru, RevistaPeruana de Biologia, 17(1): 137-140
- nMathews, D.P., Chu–Koo, F.W., Malta, J.C.O., Gomes, A.L.S.,Varella, A.M.B., Tello, M.S. (2007). Fauna ectoparasitaria en alevinos de paicheArapaima gigas (Shinz, 1822) cultivados en el centro de Investigaciones de Quistococha, Loreto, Perú, Folia Amazonica, 16(1): 23-27
- nMathews, D.P., Mathews, D.J.P., Vega, A.J., Ismiño O.R., (2011). Massive infestation by Perulernaeagamitanae(Crustacea: Cyclopoida: Lernaidae) in juvenile gamitana, cultured in the Peruvian Amazon, Veterinaria México, 42(1): 59-64
- nMathews, D.P., Mertins, O., Mathews, J.P.D., Ismiño O.R., (2013a). Massive parasitismo by Gusseviatucunarense(Platyhelminthes: Monogenea: Dactylogyridae) in fingerlins of bujurqui-tucunare cultured in the Peruvian Amazon, ActaParasitologica, 58(2): 223-225. doi: 10.2478/s11686-013-0129-7 n
- nMathews, D.P., Mathews, J.P.D., Ismiño, O.R., (2013b). Parasitic infections in juveniles of Prochilodusnigricanskept in a semi-intensive fish farm in the Peruvian Amazon, Bulletin of European Association of Fish Pathologist, 33(1): 28-32
- nMathews, D.P., Mathews, J.P.D., Ismiño, O.R., (2013c). Parasitic infections in juveniles of Arapaima gigas (Schinz, 1822) cultivated in the Peruvian Amazon, Annals of Parasitology, 59(1): 43-48
- nMendoza-Franco, E.F., Sholz, T., Roskosná, P., (2010). Tucunarellan.gen. and Other Dactylogyrids (Monogenoidea) from Cichlid Fish (Perciformes) from Peruvian Amazonia, Journal of Parasitology, 96(3):491-498.doi: 10.1645/GE-2213.1n
- nMuusze, B., Marcon, J., Thillart, V.D., Almeida-Val, V., (1998). Hypoxia tolerance of Amazon fish: respirometry and energy metabolism of the cichlid Astronotusocellatus, Comparative Biochemestry and Physiology, 120:151–156. doi: 10.1016/S1095-6433(98)10023-5 n
- nScholz, T., (1999). Parasites in culture and feral fish, Veterinary Parasitology, 84(3):317-335. doi: 10.1016/S0304-40174 n
- nSherry, J.P., (2003). The role of biomakers in the health assessment of aquatic ecosystems, Aquatic Ecosystem Health Manage, 6(4): 423-440. doi: 10.1080/714044172 n
- nThatcher, V.E., (1991). Amazon Fish Parasites, Amazoniana, 11(3): 263- 571
- nThatcher, V.E., (2006). Amazon Fish Paraites. Pensoft Publishers, Sofia-Moscow
- nVan Der Oost, R., Beyer, J., Vermeulen, N.P.E., (2003). Fish bioaccumulation and biomarkers in environmental risk assessment: a review, Environmental Toxicology and Pharmacology, 13(2): 57-149
- nVarella, A.M., Malta, J.C.O., (1995). Gamidactylushopliussp. n. (Copepoda: Peocilostomatoida: Vaiganidae) das fosasnasais, branquias de Hopliasmalabaricus(Block, 1974) (Characiformes: Erythrinidae) da Amazôniabrasileira, ActaAmazônica, 25(2): 281-288
- nVidal-Martinez, V.M., Sholz, T., Aguirre-Macedo, M.L., (2001). Dactylogyridae of Cichlid fishes from Nicaragua, Central America, with description of Gusseviaheterotilapiaesp. n. and Three New Species of Sciudicleithrum(Monogenea: Ancyrocephalinae), Comparative Parasitology, 68(1):76-86
- nYamada, F.H., Santos, L.N., Takemoto, R.M., (2011). Gill ectoparasites assemblages of two nom-native Cichla populations (Perciformes, Cichlidae) in Brazilian reservoirs, Journal of Helminthology, 85(2): 185-191. doi: 10.1017/S0022149X10000441n








