Keywords
Atlantic Lizardfish (Synodus saurus), von Bertalanffy Growth Constants, Reproduction, Mortality Rates, Exploitation Rate
Introduction
The fish fauna and their stocks that constitute a big part of biological wealth of Turkish seas have been declining due to pollution and over fishing. However, new fish species is being added to Lessepsian (Red Sea immigrant) fauna of the Mediterranean Sea each year, as a result of opening of the Suez Canal between the Mediterranean and the Red Sea. It is also reported that 59 new fish species have entered into the Mediterranean sea so far (Golani, 2002). Therefore, positive contributions have been made to Turkish fishermen’s fishing activities by these economically important fish species (Bingel, 1987). However, as Ben-Tuvia (1985) reported that immigrant Brushtooth Lizardfish (Saurida undosquamis), which is a Lessepsian species, had firstly been caught on the coast of Israel in 1952 and since then it has become one of the economically important fish species. Atlantic lizardfish (Synodus saurus) which is a member of the same family and local species to Mediterranean has almost disappeared in the catch along the coast of Israel. Bingel (1987) had realized during trawling in the northeastern Mediterranean that abundance of Brushtooth Lizardfish (Saurida undosquamis) was highest, constituting about 20- 25% of the main-catch as total biomass and also stated that Atlantic lizardfish was rarely caught with trawls in this area.
Fischer et al. (1987), Sulak (1990) and Golani (1993a, 2002) provided information about some biological characteristics and geographical distribution of Atlantic lizardfish. Ben-Tuvia (1966, 1985), Bingel (1987) and Gücü & Bingel (1994) studied the availability and fishing of this species on the coast of Israel and Turkey, respectively.
No study has been carried out about population parameters of the Atlantic lizardfish. Therefore, this study was conducted to determine the growth constants, reproduction, mortality and exploitation rates of this species inhabiting the coast of Babad?lliman? Bight (northeastern Mediterranean).
Materials and Methods
The samples used in this study were monthly obtained from trawl catches in the Babad?liman? Bight situated in the northeast of the Mediterranean Sea, between May 1999 and April 2000 (Figure 1). Individuals were caught in the depths ranging from 20 to 120 m by a commercial trawl vessel using the “Mediterranean deep trawl net” with a 22 mm cod end mesh-size and towing duration was restricted to one hour. The samples were kept in a 10% formaline solution buffered with borax in plastic barrels and transported to the laboratories.
Figure 1. Trawling stations in the Babad?lliman? Bight
Total length measurements were made with 1 mm class intervals. The body and gonad weight were measured at 0.01g sensitivity in the laboratory. Sex determination was made by examining the abdominal part of each individual after dissection. In order to estimate the spawning season, monthly mean Gonadosomatic Index (GSI) values were calculated using the formula given by Gibson & Ezzi (1978) as GSI =(GW/TW-GW)x100. Fulton’s Condition Factor (CF) was calculated using the formula given by Htun-Han (1978) as CF= W/L3x100 to assess the maturity and the condition of Atlantic lizardfish. Aging was determined through sagittal otoliths (Holden and Raitt, 1974). Age groups were formed and mean length and weight values for each age group were also calculated. Growth in length and weight was examined separately by sex and age. von Bertalanffy’s (1938) growth equation was applied to estimate the growth in length and weight. The LWR was calculated using the formula W=aLb, where ‘a’ is constant proportionality and ‘b’ is regression coefficient which were estimated by the least squares regression method. The growth type was identified by Student’s t-test using the SPSS computer program (Cicek et al., 2006)
The Regression Method suggested by Sparre and Venema (1992) was used to estimate the constants of growth in length. Any statistical difference between the mean length and weight values were compared for the measured and estimated values for each group by using the Chi-Square (X2; p>0.05) Test.
The total mortality rate (Z) was divided into its components as fishing (F) and natural mortality rates (M). The natural mortality rate was estimated by using the mean weight method which is described by Ursin (1967) as M= W- 1/b. Total mortality rate was determined by using mean age composition as suggested by Sparre et al. (1989) and (F) was estimated by using (Z) and (M). In addition to this, exploitation rate was determined by using the equation of E= F/Z given by Sparre and Venema (1992).
Results and Discussion
Age and Growth
Sex and age distributions were obtained from 161 Atlantic lizardfish which are given in Table 1. Age groups I-V for males and I-X for females were determined while age groups VII, VIII and IX were not present among the examined specimens. When age groups are taken into consideration, it was observed that male individuals indicated dominance in the age groups of III and females almost indicated equal dominance in the age groups of III, IV and V. It was found that total length of male and females ranged between 11.7-23.2 cm and 10.7-31.0 cm, respectively.
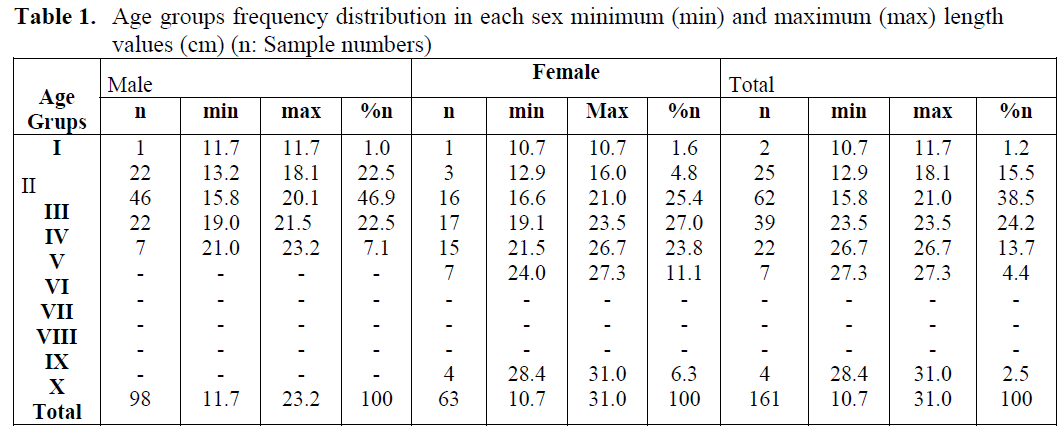
Table 1. Age groups frequency distribution in each sex minimum (min) and maximum (max) length values (cm) (n: Sample numbers)
Among 161 specimens, 60.86% were identified as males, 39.13% as females. The overall female male ratio was 1:1.5, and the χ2 test revealed that the number of males was significantly greater than that of females throughout the sampling period (χ2; p < 0.05).
von Bertalanffy growth constants in length and weight for this species are summarized in Table 2. Mean length and weight values calculated for each age group and those estimated by von Bertalanffy growth constants are given in Table 3. No statistical difference was found between the calculated and estimated length and weight values (X2; p>0.05).
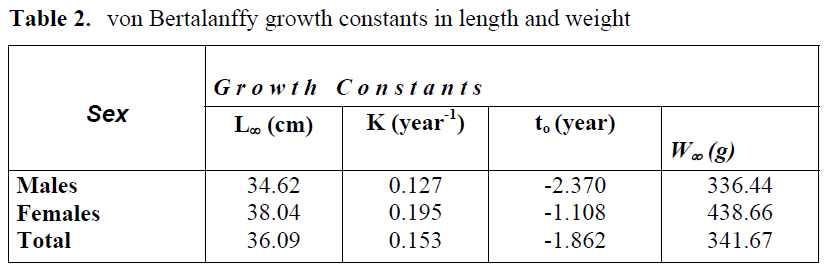
Table 2. von Bertalanffy growth constants in length and weight
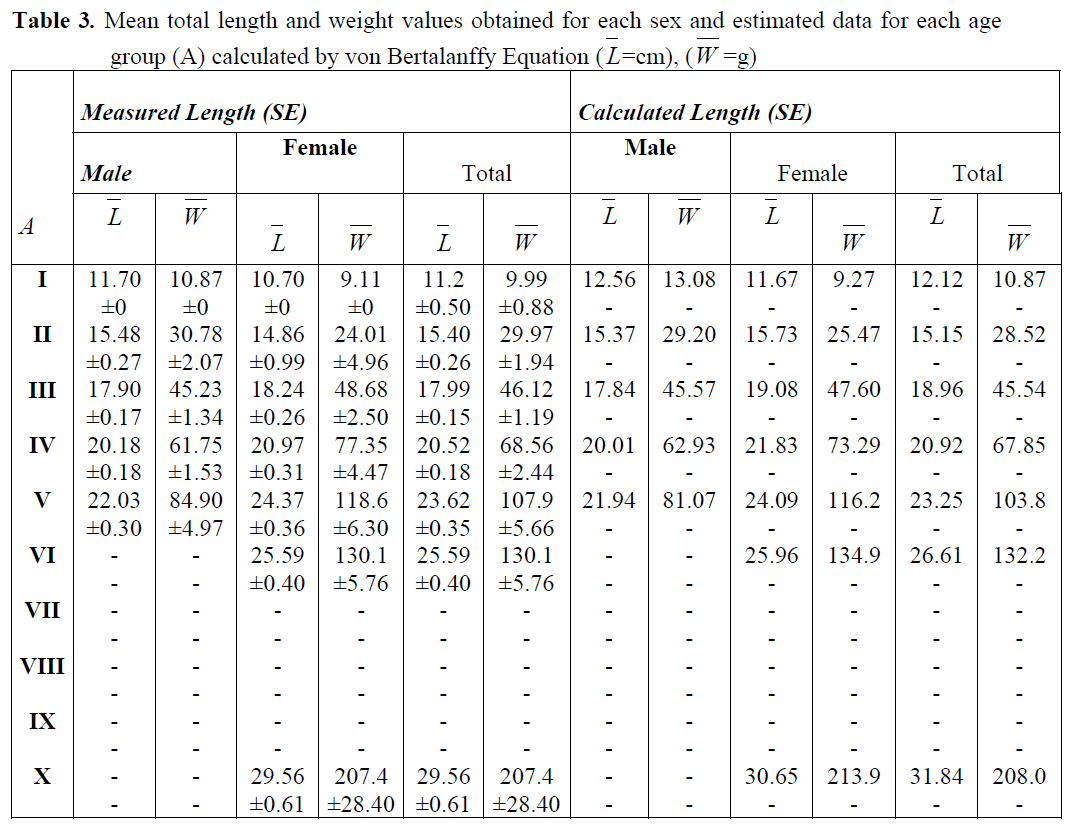
Table 3. Mean total length and weight values obtained for each sex and estimated data for each age group (A) calculated by von Bertalanffy Equation (L=cm), (W =g)
The highest increase in length of Atlantic lizardfish was found in the age groups of I and II (2.81 cm in males, 4.06 cm in females, mean=3.94 cm). The highest increase in growth as weight was found in the age groups of IV and V (18.14g for males, 42.91g for females and 35.99g for both sexes) (Table 3).
Length-Weight Relationship
Total length and weight values, which have been used for length-weight relationship, of males and females were ranged from 10.7cm, 9.99g to 31cm and 267.5g, respectively. The equations of length-weight relationship for both sexes and the total were given in Table 4.
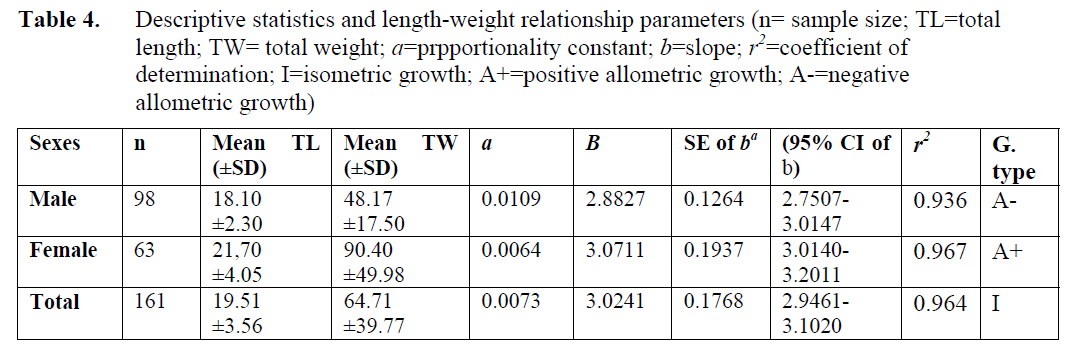
Table 4. Descriptive statistics and length-weight relationship parameters (n= sample size; TL=total length; TW= total weight; a=prpportionality constant; b=slope; r2=coefficient of determination; I=isometric growth; A+=positive allometric growth; A-=negative allometric growth)
When the regression coefficient value (b) in length-weight relationship equation was taken into account, the males of this species have shown negative allometry, which were thinner as compared to females that showed positive allometry.
Reproduction
Monthly changes of gonadosomatic index values (GSI) for each sex, (samples of November, December and January could not be obtained) are shown in Figure 2.
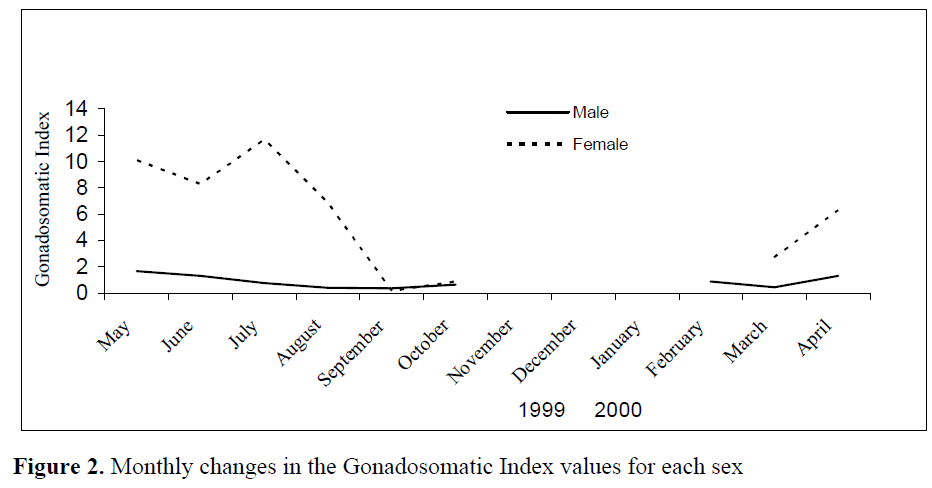
Figure 2. Monthly changes in the Gonadosomatic Index values for each sex
As it is shown from Figure 2, sexual maturation in both sexes of the Atlantic lizardfish in the Babadillimani Bight appeared to begin in March. Male individuals sexually matured in April and May and shed their sperms throughout June, August and September. However, females became fully mature in April and May, and spawned small amount of eggs in June. After the second maturing period in July, a majority of females spawned throughout August and September. Despite the fact that no samples could be obtained in November, December and January, the pattern of GSI from September onwards shows that both sexes rest during autumn and winter months.
Monthly changes of Fulton’s Condition Factor (CF) values in the year are shown in Figure 3. Monthly changes in the CF values indicates a similar trend to those of the GSI, which shows a little undulation and that fluctuation was less in males than females.
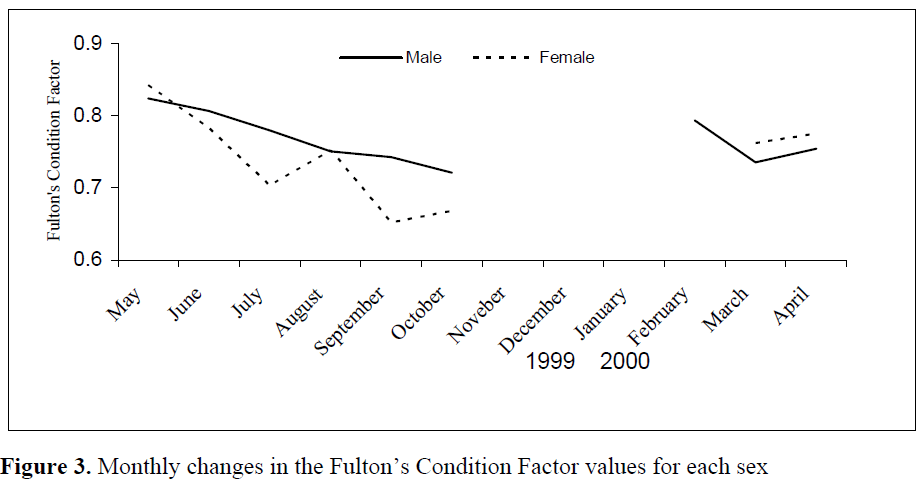
Figure 3. Monthly changes in the Fulton’s Condition Factor values for each sex
The value of the CF started to decline at the beginning of May in both sexes. After a stagnation period for male individuals in August, this decline continued until September and even October, when shedding ended. A decline in female’s condition continued until July, by which time the GSI values showed an increase, and then onwards the declining pattern continued until September.
Thus, Fulton’s Condition Factor values showed a decline (Figure 3) when eggs maturity began in April as seen from monthly GSI values in Figure 2. The decline continued between May and July when the first spawning period took place. The fish regained condition in August, but due to the most intensive spawning activities between August and September, the condition fell to the lowest level during these months. The condition started to increase again in October. The conditions of males were low between February and March probably due to the abiotic environmental factors (the Sea Surface Temperature had been measured as 16.3°C in February but 15.3°C in March during the samplings). After March, both females and males started to increase their conditions as preparations for spawning period and it can be seen that this increase in condition continued until May.
Fecundity
The mean annual fecundity of Atlantic lizardfish was calculated as 30070 ±28529.46 eggs/individual/per year from mature females of 28 individuals sampled within spawning period. Total length and weight of these individuals ranged from 16.6 to 31.0 cm, and 31.43 to 267.56g, respectively.
Mortality Rates and Level of Exploitation from the Stock
Total mortality rate (Z), natural mortality rate (M), fishing mortality rate (F) and the level of exploitation from the stock (E) for each sex and their total are given in Table 4. It was found that the natural and fishing mortality rates of the males were higher than those of the females and for both sexes. The natural mortality rates were approximately 1.5 times higher than fishing mortality of the Atlantic lizardfish.
Table 4 shows that exploitation rate for the males was optimum (E=0.5), whereas this was close to optimum for females (E=0.43). However, the total exploitation rate was not sufficient; in other words, it can be said that there has been optimum exploitation rate for males, but exploitation rate for females and total individuals is under that for this species.
The regression coefficient value “b” in length-weight relationship equation was calculated as 3.0585 for the males, 3.1693 for the females and 3.0946 for the total of the Atlantic lizardfish in the Babad?lliman? Bight. All of these values showed that the females of this species were shorter and fatter than that of the males; but the males had more fuziform bodyshape than that of the females. According to FishBase (2002), and Marella et al. (1997), the “b” value of the Atlantic lizardfish was 3.1900 for the west coast of Spain. This value is nearly equal to value calculated for the Atlantic lizardfish (3.0241) in the Babad?lliman? Bight.
The common lengths for males, females and the total of the Atlantic lizardfish in Babad?lliman? Bight were 15-19 cm and 15-21 cm, respectively. Fischer et al. (1987) indicated that the common length for this species in the Mediterranean Sea was 15-25 cm. Hence, the common length reported in present study and in that of Fischer et al. (1987) were not quite the same but similar. It might be claimed that the length of the Atlantic lizardfish in Babad?lliman? Bight is shorter than that of the others reported in the Mediterranean Sea.
The asymptotic length (L∞) for this species was reported as 43 cm (Fischer et all., 1987) and 35 cm (Ak?iray, 1987) in the Mediterranean Sea and 40 cm in the eastern Mediterranean (Sulak, 1990). However, in the current study, the asymptotic length was 36.09 cm for the males, 34.62 cm for the females and 38.04 cm for the total. These results obtained from the stock in the Babad?lliman? Bight were similar to those reported by Ak?iray (1987) for the North-eastern Mediterranean stock; but were lower than those reported by Fischer et al. (1987) for the Mediterranean Sea in general.
It was found that the exploitation rate was similar for males (0.50) and females (0.43) although fishing pressure on males was higher than that of females. Bingel and Av?ar (1988), who studied on the Lessepsian Brushtooth lizardfish (Saurida undosquamis) belonging to the same family and have similar feeding behaviour with the Atlantic lizardfish, report that females of this species feed twice more than males and are more active in the North-eastern Mediterranean Sea in any period; hence males are under more fishing pressure. In other words, as deep trawl fishing is more effective on passive species, more males were caught than females of the Atlantic Lizardfish. Therefore, it is normal to expect the exploitation rate on males to be higher than for females of the species studied in the Babad?lliman? Bight.
Conclusion
In this study, spawning periods for this species appeared to take place twice in a year namely in May-June and August-September. It might be claimed that the resting period of the Atlantic lizardfish begins in September and continues throughout autumn and winter months (Figure 2, 3). For the species, spawning period was reported to be between April and July in the Turkish coasts (Ak?iray, 1987), and between February and August in the Omman Gulf (Golani, 1993b). It appears that the spawning period for the Atlantic lizardfish is different in various geographical regions and that this might be due to different biotic and abiotic factors which prevail in the above regions.
1618
References
- Aksiray, F., (1987). TürkiyeDenizBaliklariveTayinAnahtari (Turkish Marine Fishes and Their Identification Sheets) (2. Baski) Istanbul Univ. RektörlügüYayinlari No:3490, Istanbul, 811
- Ben-Tuvia, A., (1966). Red Sea Fishes Recently Found in the Mediterranean. Copeia, 2: 254-275.
- Ben-Tuvia, A., (1985). The Impact of the Lessepsian (Sues Canal) Fish Migration on the Eastern Mediterranean Ecosystem. (Moraitan-Apostolopaulo, M. And Kiortsis, V., eds.). Mediterranean Marine Ecosystem. Plenucu Press, New-York, 367- 375.
- Beverton, R. J. H., Holt, S. J., (1957). Areview of Methods for Estimating Mortality Rates in Exploited Fish Populations, with Special Reference to sources of bias in catch sampling. Rapp. P.-v. Reun. CIEM, 140(1): 67-85.
- Bingel, F., (1987). DoguAkdeniz’deKiyiBalikçiligi Av AlanlarindaSayisalBalikçilikProjesiKesinRaporu (Final Report of the Quantitative Fishery Project in the Coastal Fishing Grounds of the Northeastern Mediterranean). Proje No: 80070011, Içel. Türkiye, 312 p
- Bingel, F., Avsar, D., (1988). Food Items of Sauridaundosquamis in the Northern Cilician Basin (Eastern Mediterranean). Rapp. Comm. Int. Mer. Medit., 31: 2
- Cicek, E., AVSAR, D., YELDAN, H., OZUTOK, M., (2006). Length-weight relationships for 31 teleost fishes caught by botom trawl net in the Babadillimani Bight (northeastern Mediterranean). J. Appl. Ichthyol, 22: 290-292.
- Fishbase., (2002). Sauridaundosquamis (Brushtooth lizardfish), https://filaman.unikiel. de/Summary/SpeciesSummary.cfm.ge nusname=Saurida&speciesname=undosquamis, 03/07/2002.
- Fischer, W., Bauchot, M. L., Schneider, M., (1987). Fisches FAO D’identification des EspecesPoun les Besoins de la Peche (Revision 1). MediterraneeetMer Noire. Zone de Peche 37. Volume II. Vertebres. Publication Preperee la Fao, Resultatd’unAccord Entre la FAO et la Commision des CommunautesEuropeennes (Projet GCP/INT/442/EEC) FinanceeConjointement Per CesDeux Organisation. Rome, FAO, 2: 761-1530.
- Gibson, R.N., Ezzi, I.A., (1978). The Biology of a Scotfish Population of Fries’ goby, Lesueurigobiusfriesii. J. Fish. Biol. Vol. 17: 371-389.
- Golani, D., (1993a). Trophic Adaptation of Red Sea Fishes to the Eastern Mediterranean. Enviromen-Review and New Data Israel Journal of Zoology. 39: 391-402.
- Golani, D., (1993b). The Biology of the Red Sea Migrant, Sauridaundosquamis, in the Mediterranean and comparison with the Indigenous ConfamilialSynodussaurus (Teleostei: Synodontidae). Hydrobiologia, 271: 109-117.
- Golani, D., (2002). Lessepsian Fish Migration- Characterization and Impact on the Eastern Mediterranean. Workshop on Lessepsian Migration, 20-21 July Gökçeada-Turkey, 1-9.
- Gücü, A.C., Bingel. F., (1994). Trawlable Species Essemblages on the Continental Shelf of the Northeastern Levant Sea (Mediterranean) with an Emphasiss on Lesseptian Migration. ActaAdriat. 35(1/2): 83-100.
- Holden, M.J., Raitt, D.F.S., (1974). Manual of Fisheries Science. Part 2- Methods of Recourse Investigation and their Application. FAO Fish. Tech. Pap., (115). Rev. 1: 214.
- Htun-Han, M., (1978). Reproductivite Biology of the Dab Limandalimanda (L) in the North Sea: Gonadosomatic Index, Hepasomatic Index and Condition Factor. J. Fish. Biol. 13: Sci. No. 1: 351-377.
- Ricker, W.E.., (1975). Computation and Interpretation of Biological Statistics of Fish Population. Bull. Fish. Res. Board Can. 191: 382.
- Sparre, P., Ursin, E., Venema, S.C., (1989). Introduction to tropical fish stock assessment Part -Manual. FAO Fish. Tech. Pap. No 306.1. Rome, FAO, 337 p.
- Sparre, P., Venema, S.C., (1992). Introduction to Tropikal Fish Stok Assessment. Part 1. Manual. FAO Fish. Tech. Pap. No. 306. 1. Rev. 1. Rome, 376.
- Sulak, K.J., (1990). Synodontidae.In J.C. Quero, J.C. Hureau, C. Karrer, A. Post and L. Saldanha (eds.) Check-list of the Fishes of the Eastern Tropical Atlantic (CLOFETA). JNICT, Lisbon; SEI, Paris; and UNESCO, Paris. Vol. 1. 365-375.
- v. Bertalanffy, L., (1938). A quantitave Theory of Organic Growth. Hum. Biol. 10: 181- 213.
- Ursin, E.A., (1967). Mathematical Model of Same Aspects of Fish Growth, Respiration and Mortality. J. Fish. Res. Board Can., Bull. 90: 141-147.













