Keywords
Cuttlefish, Sepia officinalis, reproduction, fecundity, Aegean Sea
Introduction
The common cuttlefish, Sepia officinalis Lin-naeus, 1758, is a demersal, neritic species occur-ring predominantly on sandy to muddy bottoms from the coastline to about 200 m depth, but most abundant in the upper 100 m; larger individuals are encountered in the deeper part of the range. The geographical distribution of this species is ranged from the Baltic and North Seas to South Africa and Mediterranean Sea (Roper et al., 1984).
The cuttlefish, like most cephalopods, is a short-lived species which reproduces only once over a short period at the end of its life (Mangold, 1987). Roper et al. (1984) reported that the re-production period of common cuttlefish occurs throughout the year, with peaks at water temper-atures from 13º to 15ºC in the western Mediterra-nean, between April and July, off Senegal and on the Sahara Banks between January and April. On the latest a minor spawning peak of medium and small-sized individuals in late summer and early autumn has been reported.
According to Rocha et al. (2001), common cuttlefish has a reproductive strategy and an in-termittent spawning, dying after spawning. The species displays monocyclic spawning, i.e. egg-laying occurs in separate batches. Arkhipkin (1992) stated that the reproductive strategies of cuttlefish were different from those of octopus. According to the same author, cuttlefish is a mainly necto-benthic animal which accumulates eggs in the coelom similarly to octopus. None-theless, before spawning the eggs are covered with oviducal gland secretions and also with a thick capsule secreted by the nidamental glands. Eggs are laid one by one or in clusters in differ-ent kinds of shelters or on hard substrates.
The cuttlefish is relatively abundant species in the Aegean Sea and they are heavily exploited for their high market value in Turkey. There has been an exponential increase in the landings of cuttlefish by trawlers, jigging liners and coastal trammel netters from Turkish Aegean Sea for a long time. Annual landings have increased from about 300 t in 1972 to over 1220 t in 2005; with the peak of 5416 t in 1989 (FAO, 2000). In Izmir Bay, cuttlefish fishery is carried out by using jig-ging line, trammel net and fyke net in inshore waters. This area has been closed to trawlers and purse-seiners for along time.
Although, there is some data regarding on the reproductive biology of cuttlefish all over the world (Roper et al., 1984; Mangold, 1987; Ark-hipkin, 1992; Forsythe et al., 1994; Gauvrit et al., 1997; Dunn, 1999; Hanlon et al., 1999; Rocha et al., 2001; Sykes et al., 2006), there is a gap for knowledge in the Aegean Sea. Until now, only two reproduction studies (Laptikhovsky et al., 2003; Önsoy and Salman, 2005) on the cuttlefish in the Aegean Sea were performed.
This study aims at providing new data on some reproduction aspects of S .officinalis, such as sex ratio, maturation, reproduction period and fecundity, and to compare it with the previous studies in the same region.
Materials and Methods
Monthly samples of cuttlefish, S. officinalis, were collected from the commercial trammel netters of Izmir Bay (Aegean Sea, Turkey) from November 2006 to November 2007 (Figure 1). However, we could not find any cuttlefish in two months, July and August.

Figure 1. Sampling area
A total of 333 specimens, 248 males, 81 fe-males and 4 unidentified sex, were sampled (12 in December, 70 in January, 18 in February, 13 in March, 13 in April, 34 in May, 37 in June, 40 in September, 79 in October, 17 in November) and analyzed in the laboratory for detailed biological studies on the species. Total body weights (BW) and dorsal mantle lengths (ML) were measured in fresh animals to the nearest 0.1 g and 0.1 cm, re-spectively. A total of 329 ovary weight from both sexes and 1964 egg diameters from females were estimated to the nearest 0.01 g and 0.01 mm, re-spectively. The means were given with standard error (± SE).
Maturity stages were assigned according to Arkhipkin (1992). The stages were assumed as follows: 0, juvenile; I and II, maturing gonads; III, mature gonad in both sexes, but we did not find any samples of egg and spermatozoa in the coelom (i.e. stage IV). The gonads were then stored in 4% formaldehyde.
The sex ratio was calculated monthly and an-nually. Significant deviations from the 1:1 sex ratio were tested by the χ2-test (α=0.05).
Gonadosomatic index for both sexes were determined by the following equation: GSI=(OW/BW)x100, where OW is ovary weight.
The observed total oocyte stock was assumed to represent fecundity. The fecundity was gravi-metrically estimated from the ovaries of 49 ma-ture females, with a range of sizes and weights from 8.9 to 19.5 cm ML and from 131.4 to 959.5 g BW, respectively.
Results and Discussion
The ML and BW values of cuttlefish in all in-dividuals were between 5.5 and 22.4 cm with an average of 11.6 ±0.16 cm, and between 25 and 1189 g with an average of 249.8 ±10.4 g, respec-tively. The length frequency has shown that the 11-12 cm length class had the highest number of specimens (Figure 2).
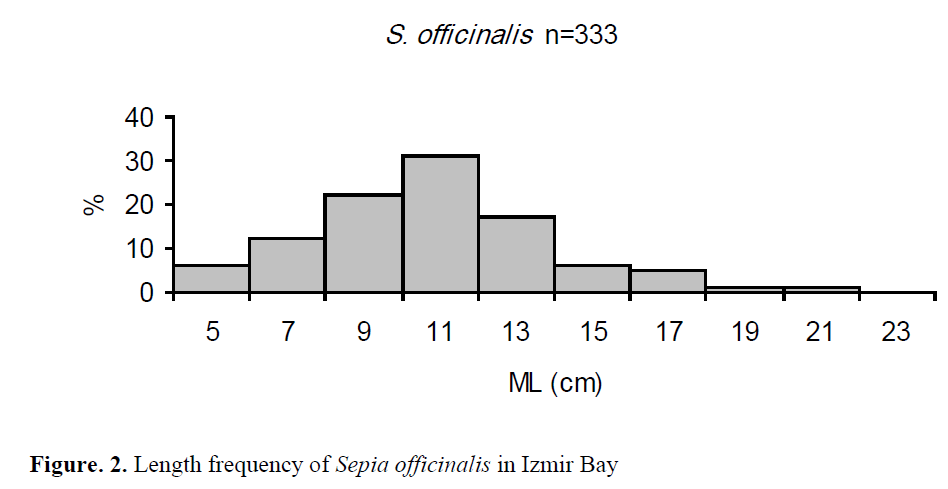
Figure. 2. Length frequency of Sepia officinalis in Izmir Bay
The annual sex ratio (females: males) of the sampled specimens was 1:3.1. The application of the χ2-test showed that this difference was sig-nificant (P<0.05), even when analyzing the sex ratio by month.
Monthly evolution of the maturity stages of males and females during the sampled period is shown in Figure 3. Mature population was observed in all sampled months for females, but stage III (mature gonad) for males was only seen in June. The completely mature females (100%) were monthly recorded between February and October. However, there was no female in the juvenile stage (0), whereas a total of 37 males were in the juvenile stage. The first maturing and/or mature stages (II and III), based on the 50% of total samples in the population were observed in 80 mm ML for females and in 90 cm ML for males (Table 1).
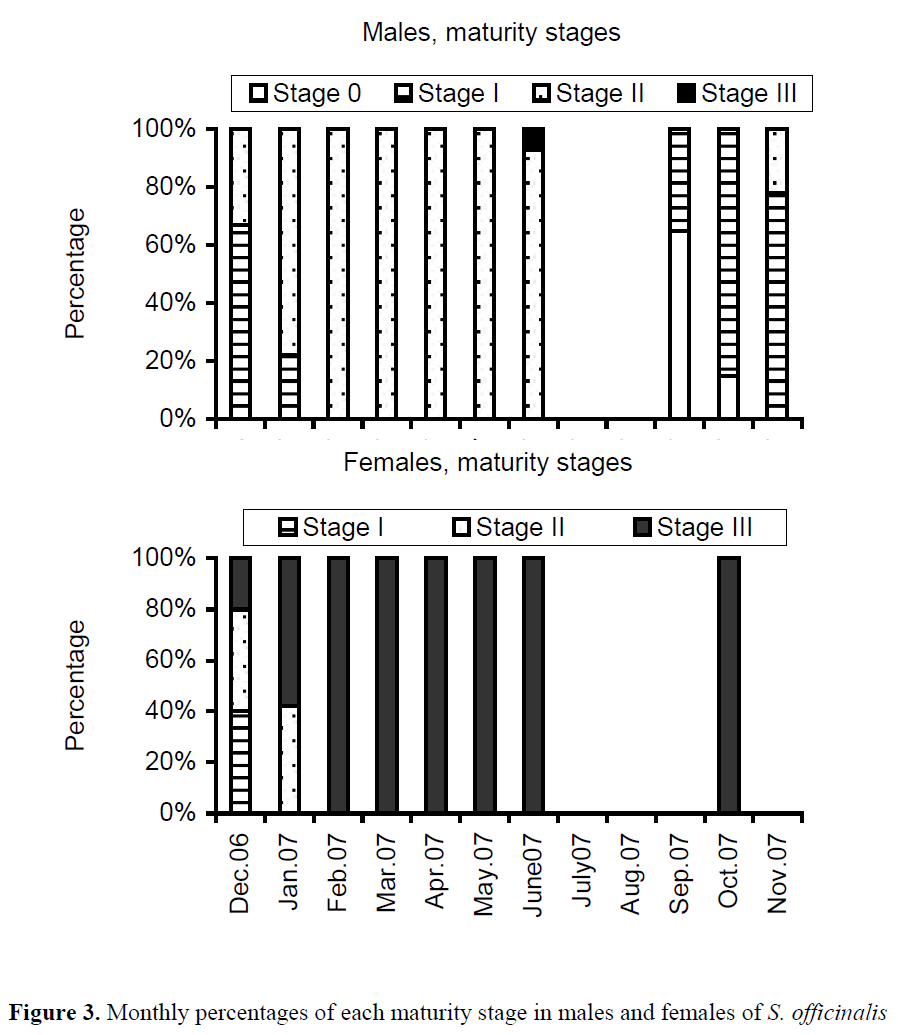
Figure 3. Monthly percentages of each maturity stage in males and females of S. officinalis
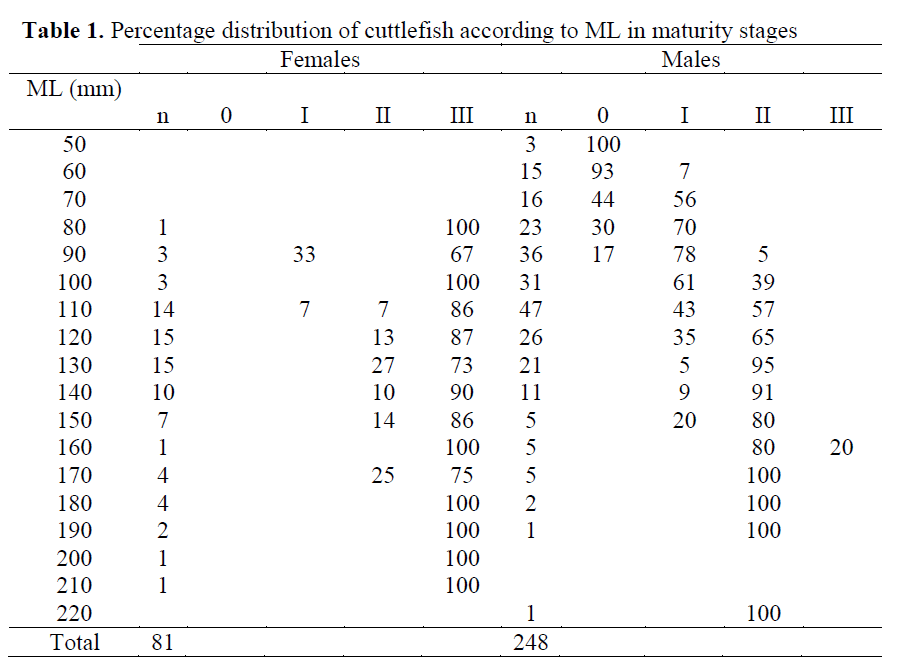
Table 1. Percentage distribution of cuttlefish according to ML in maturity stages
The monthly GSI results indicated that the re-productive season of S. officinalis ranged from March to October, with a maximal GSI of 5.64 in May for females and December to February and April, with a maximal GSI of 2.32 in April for males (Figure 4).
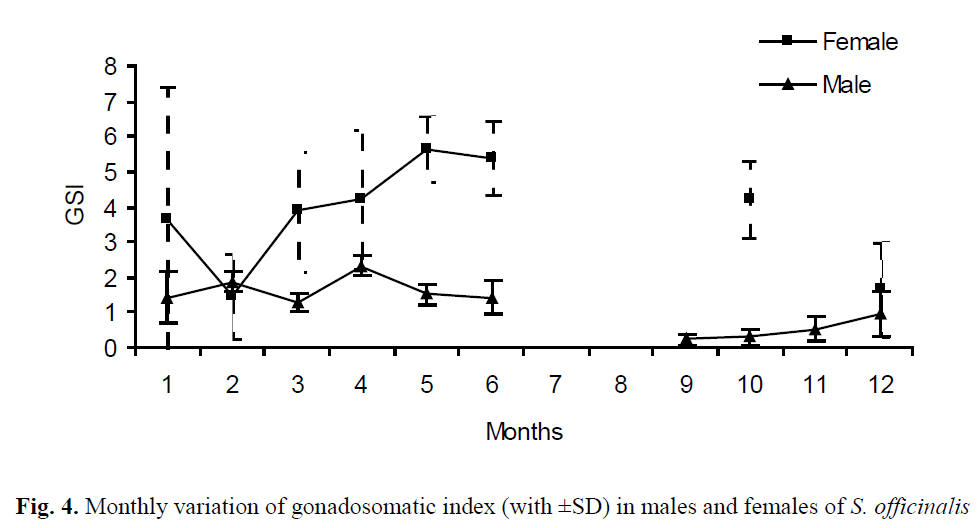
Figure. 4. Monthly variation of gonadosomatic index (with ±SD) in males and females of S. officinalis
The mean diameter (long axis) of the oocytes extracted from 49 females was 5.21 mm ±0.03 and ranged from 1.91 mm to 9.44 mm (Figure 5). Total number of oocytes varied from 49 to 828 with an average of 261 ±27 per specimen. The relationship between ML, BW and number of oo-cytes in S. officinalis is shown in Figs. 6, 7. There was a low correlation in the regressions.
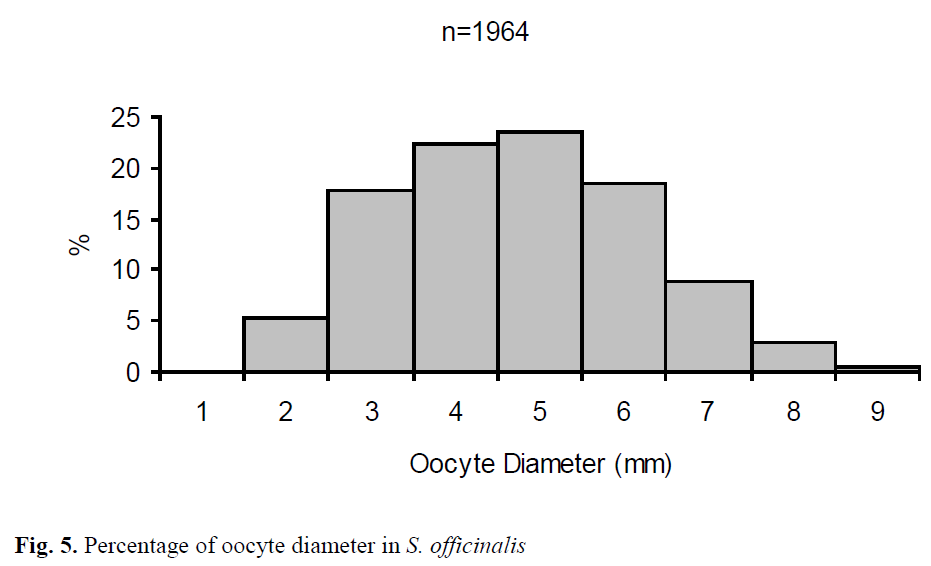
Figure 5. Percentage of oocyte diameter in S. officinalis
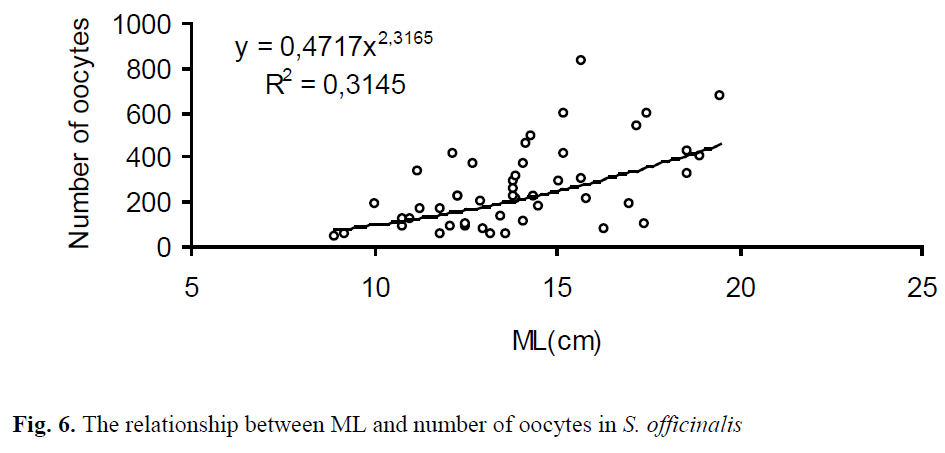
Figure 6. The relationship between ML and number of oocytes in S. officinalis
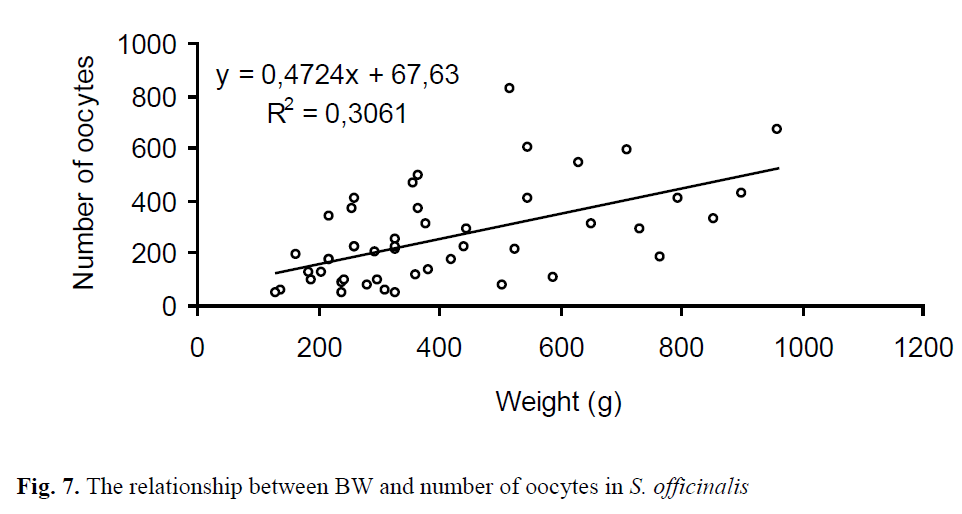
Figure. 7. The relationship between BW and number of oocytes in S. officinalis
In the present study, any cuttlefish could not be found in the dock samplings in two months, July and August. According to fishermen from trammel netters, the cuttlefish normally disap-pears in these two months since they move to deeper zones. So, they don’t want to use their trammel nets in the deeper zone due to have dif-ficulty in hauling of the net.
In this study, the ML and BW values of cuttle-fish in all individuals were between 5.5 and 22.4 cm with an average of 11.6 ±0.16 cm, and be-tween 25 and 1189 g with an average of 249.8 ±10.4 g, respectively. They were peaked between 11 and 12 cm length class. Önsoy and Salman (2005) reported that the length range of the spe-cies in Homa Lagoon (Izmir Bay) samples were between 6.3 to 24.1 cm for both sexes. Many of the caught cuttlefish in the study were smaller sized specimens. Moreover, 71% (this study) and 63% (calculated from Önsoy and Salman, 2005 from cuttlefish captured at the Bay of Izmir) of the samples were <12 cm in ML size. According to Roper et al., (1984), the maximum ML of S. officinalis was 45 cm, weight up to 4 kg in tem-perate waters, but only little more than 30 cm and 2 kg in subtropical seas. Common sizes in the West Saharan fisheries ranged between 15 and 25 cm. And also, off Tunisia, first maturity length was 12 cm in females and 10 cm in males (Roper et al., 1984). The smaller size of cuttlefish in this study is indicatory of the over-fishing on the spe-cies in the Aegean Sea. However, the smaller specimens additionally shown that the Bay of Izmir is a nursery and reproduction area for the species. Although, we never encountered the maturity stage IV for both sexes in the bay, many cuttlefish’s egg clusters were found on macro-alga, Gracilaria sp. on the coast of Urla, Izmir (at a depth of 0.5 m) in the beginning of March (H. ?en, pers. comm.). Subsequently, a total of 1567 eggs laid on the air-pipe in a tank at 32 times in-tervals during 46 days (mean water temperature: 17.4 ±1.1ºC) between March and May from five wild cuttlefishes under controlled conditions (?en, 2009).
Annual sex ratio showed that the males were predominant, whereas females were recorded with surpass (F:M ratio = 1:0.93) in Homa La-goon for year 2000-2002 samplings (Önsoy and Salman, 2005). Mangold-Wirz (1963) and Man-gold (1983) stated that the reproductive migra-tions affected the sex ratio in relation to size in particular areas and seasons. In this study, there was a little number of female cuttlefish during summer and autumn period that they likely mi-grate for reproduction purposes.
In the present study, the first maturing/mature stages (II-III), based on the 50% of total samples in the population were observed in 80 mm ML for females and in 110 mm ML for males, whe-reas the first maturing stages were determined in 70 mm ML for females and 90 mm ML accord-ing to Önsoy and Salman (2005). Though, these results were being closed, the some differentia-tion might be arisen due to the larger samples, more observations on all of maturity stages (I-V) by Önsoy and Salman (2005). The maturity stages and GSI showed that the reproduction ac-tivity of cuttlefish is carried out during the whole year. According to GSI values, the reproduction period of female and male specimens were be-tween March and October, with a peak in May and between December and April, with two peaks (February and April), respectively. Önsoy and Salman (2005) determined that the reproduc-tion period of cuttlefish was similar in nearby of Homa Lagoon (Izmir Bay). However, though ab-sence of GSI value in July, August and Septem-ber, it is obvious that this period for females is extended with a slight decrease until October in our study. In this respect, it needs further studies on reproduction period in the area. Roper et al. (1984) reported that the reproduction period was between April and July in the western Mediterra-nean for primarily big adults and there was a se-cond minor spawning peak of medium and small-sized individuals in late summer and early au-tumn. In that case, the quantity of medium and small-sized specimens caused the extension of the period in this study.
Total oocyte stock varied from 49 to 828 with a mean of 261 ±27 oocytes and is completely in agreement with the findings of Mangold-Wirz (1963) who obtained 200-550 oocytes and of Laptikhovsky et al. (2003) who estimated 130-839 large yolk oocytes, whereas the potential fe-cundity of pre-spawning animals was much higher (varied from 3700 to 8000, mean 5871). In addition, Sykes et al. (2006) reported that the to-tal and individual fecundity data for cultured S. officinalis ranged from 586 to 5916 and from 247 to 478 eggs female-1, respectively.
In this study, there were low correlations be-tween the fecundity of the females and both ML (R2=0.31) and BW (R2=0.31). This may con-nected with maturing females, not ready to spawn yet. Laptikhovsky et al. (2003) determined that there were negative correlations unexpectedly both in ML (r=-0.06) and in BW (r=-0.18) in 29 females, ranged from 9.4 cm ML (100 g BW) to 24.7 cm ML (1908 g BW). This phenomenon may be related to the intermittent spawning of the species. According to Rocha et al. (2001), in this type of spawning, group-synchronous ovulation occurs in the ovary. Spawning is monocyclic and egg-laying occurs in separate batches during the spawning period, which is usually relatively long. So, the spawned batches may cause the negative correlation in investigation of Laptikhovsky et al. (2003).
The oocyte size in mature females ranged from 1.91 mm to 9.44 mm, with a mean of 5.21 ±0.03 mm. Many oocytes were heaped up be-tween 3 and 6 mm. Laptikhovsky et al. (2003) also reported that the smallest oocyte size in im-mature females was 0.1 mm, whereas maturing and mature females it was 0.2-0.3 mm. However, ripe egg lengths varied from 6.45 to 7.53 mm.
Conclusions
In conclusion, female cuttlefish in Aegean Sea (Izmir Bay) shows stage III maturation in all sea-sons, especially between March and October. S. officinalis has different sex ratio and the relation-ships between the fecundity and ML/BW while showing similar oocyte size, large oocyte stocks and ML frequencies with the other studies in the Aegean Sea. Further studies on the life cycle and the biology of cuttlefish should be conducted for sustaining their stocks and regulating their fish-ery.
771
References
- nArkhipkin, A.I., (1992). Reproductive system structure, development and fuction in ceph-alopods with a new general scale for ma-turity stages, Journal of Northwest Atlantic Fishery Science, 12: 63-74. doi:10.2960/J.v12.a7
- nDunn, M.R., (1999). Aspects of the stock dy-namics and exploitation of cuttlefish, Sepia officinalis(Linnaeus, 1758), in the English Channel, Fisheries Research, 40: 277-293. doi:10.1016/S0165-7836(98)00223-9
- nFAO, (2000). Fishstat Plus: Universal Software for Fishery Statistical Time Series, Version 2.3, Total Fishery Production 1950-2008 (Release date: March 2010) (2.4 Mb), FAO Fisheries Department, Data and Statistics Unit, Rome
- nForsythe, J.W., Derusha, R.H., Hanlon, R.T., (1994). Growth, reproduction and life span of Sepia officinalis(Cephalopoda: Mollusca) cultured through seven consequtive genera-tions, Journal of Zoology, 233: 175-192.
- nGauvrit, E., Le Goff, R., Daguzan, J., (1997). Reproductive cycle of the cuttlefish, Sepia officinalis(L.) in the northern part of the Bay of Biscay, Journal of Molluscan Stud-ies, 63: 19-28. doi:10.1093/mollus/63.1.19
- nHanlon, R.T., Ament, S.A., Gabr, H., (1999). Be-havioral aspects of sperm competition in cuttlefish, Sepia officinalis(Sepioidae: Cephalopoda), Marine Biology, 134: 719-728.doi:10.1007/s002270050588
- nLaptikhovsky, V., Salman, A., Önsoy, B., Katagan, T., (2003). Fecundity of the com-mon cuttlefish, Sepia officinalisL. (Cepha-lopoda, Sepiida): a new look at an old prob-lem, Scientia Marina, 67(3): 279-284
- nMangold, K., (1983). Eledonemoschata. In P.R. Boyle, Ed., Cephalopod life cycles, Vol. I. Academic Press, London, p. 387-400
- nMangold, K., (1987). Reproduction. In P.R. Boyle, Ed., Cephalopod life cycles, Vol. II.Academic Pres, London, p. 157-200
- nMangold-Wirz, K., (1963). Biologie des cephalo-pods benthiquesetnectoniques de la Mer Catalane, Vie et Milieu, Suppl.13: 1-285.
- nÖnsoy, B., Salman, A., (2005). Reproductive bi-ology of the common cuttlefish Sepia offici-nalisL. (Sepiida: Cephalopoda) in the Ae-gean Sea, Turkish Journal of Veterinary and Animal Science, 29: 613-619
- nRocha, F., Guerra, A., Gonzales, A.F., (2001). A review of reproductive strategies in cephalo-pods, Biological Reviews, 76: 291-304. doi:10.1017/S1464793101005681
- nRoper, C.F.E., Sweeney, M.J., Nauen, C.E., (1984). FAO Species Catalogue. Cephalo-pods of the World, FAO Fisheries Synopsis, 3: 1-277
- nŞen, H., (2009). Spawning, egg development and incubation of cuttlefish (Sepia officinalisL., 1758) in conrolled conditions, Journal of FisheriesSciences.com, 3(3): 169180.doi:10.3153/jfscom.2009021
- nSykes, A.V., Domingues, P.M., Andrade, J.P., (2006). Effects of using live grass shrimp (Palaemonetesvarians) as the only source of food for the culture of cuttlefish, Sepia officinalis(Linnaeus, 1758), Aquaculture International, 14: 551-568. doi:10.1007/s10499-006-9054-1














