Keywords
Habitat warming, Fish physiology, Fish growth, Oxidative metabolism, Thermal stress
Introduction
Fish belong to a paraphyletic group of organisms that consist of all gill-bearing aquatic craniates that lack limbs with digits. Fish as the major group of poikilotherms can be considered as the principal targets under rise in acute or gradual temperature of their natural habitat because these organisms lack proper physiological mechanism(s) to regulate their internal body heat in relation to changing environmental temperature (Carey and Lawson, 1973; Goldman, 1997). Consequently, their energy metabolism may be affected by increasing environmental temperature as observed in other poikilotherms (Galli and Rechards, 2012). It may result in increase in production ofreactive oxygen species (ROS) either due to elevated metabolism or due to collapse of antioxidant defences, or bothwhich in turn may induce oxidative stress (OS) in fish by impairing functions of biomolecules as a consequence of their oxidation. Oxidative stress is reported to be associated with various physiological aspects of aerobes such as growth, development and aging. Fish exhibit a great diversity in ecology and serve as a vital component in aquatic ecosystem. Also it is an important trophic level in global food chain. Therefore, increase in habitat temperature due to global warming may induce OS in fish and may hamper their growth, reproduction and aging which may indirectly affect the global ecology. This is the central focus of this perspective article.
Oxygen Metabolism and Oxidative Stress
The molecular story of oxidative stress commences with consumption of O2 and oxidation of nutrient molecules to produce energy in aerobes. In the process, mitochondria play a central role. Electron transport chain of mitochondria carries electrons from universal electron donors such as FADH2 and NADH to O2 via enzyme complexes I, II, III and IV and produces ATP in complex V (Lehninger et al., 2008). Incomplete reduction of oxygen molecules and subsequent leaking of electrons in complexes I and III during electron transport, results in formation of superoxide radicals (one of the ROS). Consequently, it leads to formation of other ROS such as hydrogen peroxide and hydroxyl radicals by classical Haeber-Weiss reaction. Cells are equipped with efficient antioxidant defences to neutralize the ROS before they can damage biomolecules by oxidation (Halliwell and Gutteridge, 2008). However, due to decrease in efficiency of antioxidant defenses in response to internal or external insults, generation of ROS are elevated which in turn oxidizes all biomolecules present in their vicinity and causes oxidative stress in cell. Under such conditions, a deviation in normal physiological functions can not be ruled out which may end up with increase in ratio between energy demand versus production, cell senescence, aging, metabolic depression, retarded growth and reproduction, and finally death may also occurs (Halliwell and Gutteridge, 2008). Therefore, careful and planned study on oxidative stress indices and redox regulatory parameters in silico and in vivo conditions will have immense importance to solve various riddles observed in several core evolutionary concepts of animal biology such as in life history tradeoffs, senescence, growth, reproduction and sexual selection in free ranging organisms (Costantini et al., 2010; Paital et al., 2011, 2013, 2015; Paital and Chainy, 2012; Paital and Samanta, 2013, Paital and Chainy, 2014; Paital and Chainy, 2010). Noteworthy, ROS are also reported to be useful because at lower concentrations, they mediate several signal transduction processes in cells (Passos and Zglinicki, 2006; Takano et al., 2003). However, under elevated thermal stress, maintenance of the nominal amount of ROS to regulate signal transduction processes is not ensured (Passos and Zglinicki, 2006).
Aerobes are equipped with both enzymatic as well as nonenzymatic redox regulatory molecules to counteract the over production of ROS to maintain redox homeostasis. Superoxide dismutase (SOD), the first enzyme of enzymatic antioxidant defense, dismutates the toxic superoxide radicals to H2O2 and molecular oxygen. H2O2 is further neutralized by two cellular enzymes, catalase (CAT) and glutathione peroxidase (GPx). CAT breaks down H2O2 to H2O and O2 while GPx reduces H2O2 and organic hydroperoxides to H2O and other non-reactive metabolites at the cost of oxidation of a reduced glutathione (GSH) molecule. The oxidized glutathione is reduced back to GSH by the enzyme glutathione reductase (GR) with the help of the reduced nicotinamide adenine dinucleotide phosphate (NADPH) (Figure 1). Peroxiredoxins are a group of ubiquitous antioxidant enzymes that regulate the levels of cytokine-induced peroxides (Rhee et al., 2005). The oxidized form of peroxiredoxins is non-catalytic in nature. To self recharge after reducing H2O2, these enzymes require thioredoxin (Rhee et al., 2001). They require electrons from the reduced thioredoxin to restore their enzymatic catalytic function (Pillay et al., 2009). Thioredoxins are a class of small ubiquitous redox proteins which also play a key role in removing ROS (Wollman et al., 1988; Meng et al., 2010). Glutaredoxins are a group of small redox enzymes which confer their antioxidant activity by reducing dehydroascorbate, peroxiredoxins and methionine sulfoxide reductase (Nordberg and Arnér, 2001). Non enzymatic antioxidant defence system comprises of small molecules such as polyphenols, carotenoids, flavonoids, ascorbic acid, vitamin E and GSH which directly scavenge ROS. Under thermal stress, the levels of redox regulatory molecules are found to be alleviated or insufficiently enhanced which fails to combat OS in organisms (Halliwell and Gutteridge, 2008).
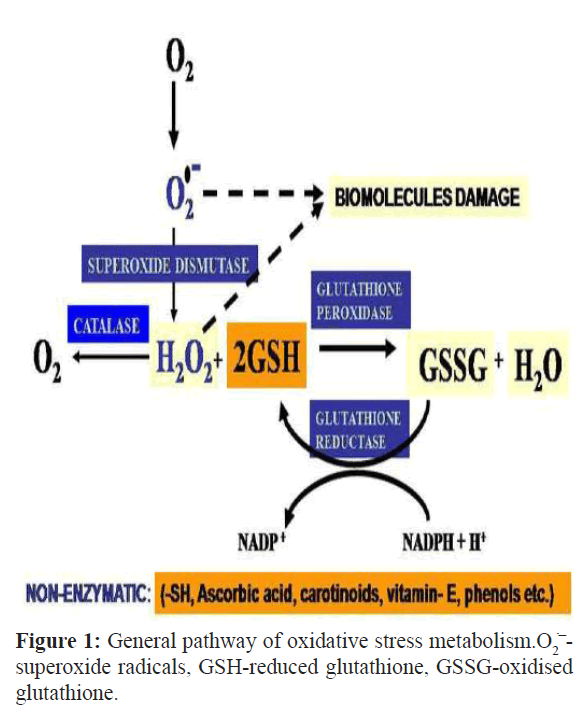
Figure 1: General pathway of oxidative stress metabolism.O2‾ superoxide radicals, GSH-reduced glutathione, GSSG-oxidised glutathione.
The Central Remark
Although not much experimental information is available on positive correlation between rise in temperature and reproduction or growth in fishes in their natural habitat, it is suggested that rise in temperature in combination with elevated CO2 level decrease the duration of nymphal stadia, the longevity and reproductive success of the aphid Sipha flava (Auad et al., 2012). Similarly, several authors have pointed out that increase in habitat temperature above 20-24ºC, which is the optimum temperature required for the growth of aphids, can negatively influence fertility, reproduction, development, life expectancy, survival and abundance of aphids (Oliveira et al., 2009; Auad et al., 2009). A common reason for the above phenomenon is provided that increase in temperature can affect the life cycle of poikilotherms directly by influencing their physiology (Flynn et al., 2006). Occurrence of the above multiple processes as the consequences of climatic changes are not only restricted to terrestrial ecosystems but also can affect the aquatic ecosystems. For example, in aquatic environment especially in coastal marine bodies, climatic changes also modulate elevate the oxidative stress and its related metabolism as well as negatively modulate the other physiological processes such as reproduction, excretion, respiration of the inhabiting ectotherms (Lawrence and Soame, 2004; Paital and Chainy, 2010, 2012; Abele et al., 2002, 2007, 2011).For examples, increase in salinity can induce oxidative stress despite altered redox status found to be initiated to protect poikilotherms (Paital and Chainy, 2010, 2012). With altered redox regulation, fish are also found to experience OS induced by rise in habitat temperature in their natural environment. For example, in cichlid fish acará (Geophagus brasiliensis), three-spined stickleback fish (Gasterosteus aculeatus), Senegal sole fish (Solea senegalensis), environmental rise in temperature induces OS (Table 1). It has been noticed that antioxidant defence parameters changes in fish brain and muscle tissues (Heteropneustus fossilis) in response to air exposure due to alteration in functional capacity of electron transport chain (Paital, 2013, 2014). Temperature is known to affect oxygen content of water bodies. Therefore, molecular and cellular functions of fish due to elevated temperature as a consequence of global warming cannot be ignored.
| Name of the Animal |
Modulator |
Tissue/organ/cell fractions |
OS |
AOE |
Reference |
Geophagusbrasiliensis
(cichlid
fish acará) |
Comparatively high temperature in spring |
Liver |
TBARS↑, GSSG↑ |
SOD↓,
CAT |
Filhoet al., 2001 |
Gasterosteusaculeatus L.
(three-spined stickleback
Fish) |
High temperature in summer with reproductive activity |
Liver |
TBARS↑ |
GPx↓ |
Sanchez et al., 2008 |
Lepomismacrochirus
(bluegill fish) |
Hightemperature in summer |
Whole animal |
NA |
NA |
Wohlschlag and Juliano, 2003 |
| Soleasenegalensis(Senegal sole fish) |
High temperature with heavy metal load |
Liver |
TBARS↑ |
CAT↓, GPx↓ |
Olivaet al., 2012 |
| Barbusbarbus L. (barbell fish) |
High temperature in summer |
Liver |
NA |
SOD↓, CAT↑ |
Radovanovicet al., 2010 |
| Barbusbarbus L. (barbell fish) |
High temperature in summer |
Muscle |
NA |
SOD↓, CAT↑ |
Radovanovicet al., 2010 |
ROS-reactive oxygen species, OS-oxidative stress (TBARS-thiobarbituric acid reactive substances, PC-protein carbonylation), AOEantioxidant enzymes (SOD-superoxide dismutase, CAT-catalase, GPx-glutathione peroxidase), GSSG- oxidised glutathione, NA- not analysed. ↓ or ↑ symbols are used to indicate decrease or increase of the parameters with the corresponding season, respectively. Parameter with no symbol (↓ or ↑) indicates “no change” in the same with respect to the season.
Table 1: Oxidative stress and redox status in natural population of some fish under high habitat temperature.
Conclusion
The present study has clearly demonstrated that the use of probiotic and nitrifying bacterial consortium in shrimp culture at laboratory scale experimental conditions increased the shrimp survival and reduced the ammonia and nitrite toxicity. Based on the water quality parameters and shrimp survival (%), it is concluded that the tank 3 (Consortium of Bacillus stratosphericus (AMET1601), Nitrosomonas sp AMETNM01 and Nitrobacter sp AMETNB03) was found to be superior as compared to other two tanks (1 and 2). The work also suggests that, the extrapolation of the present study in fields and the use of these beneficial bacterial strains in shrimp culture will definitely prevent the aquaculture ponds from undergoing eutrophication and can control the microbial diseases to the shrimps and enhance their production in an ecofriendly ambience without antibiotics but with probiotics and nitrifiers.
Acknowledgements
BRP is grateful to the Director, College of Basic Science and Humanities and Head, Department of Zoology, College of Basic Science and Humanities, Orissa University of Agriculture and Technology for their constant support to conduct research.
8212
References
- Abele, D., Heise, K., Pörtner, H.O., and Puntarulo, S. (2002). Temperature-dependence of mitochondrial functions and production of reactive oxygen species in the intertidal mud clam Mya arenaria were recorded. Exp. Biol. 205, 1831-41.
- Abele, D., Philipp, E., Gonzalez, P.M., and Puntarulo, S. (2007). Marine invertebrate mitochondria and oxidative stress. Front. Biosci. 12, 933-946.
- Abele, D., Vazquez-Medina, J.P., and Zenteno-Savin, T. (2011). Oxidative Stress in Aquatic Ecosystems, first ed. Blackwell and Wiley, USA, pp 548.
- Auad, A.M., Alves, S.O., Carvalho, C.A., Silva, D.M., Resende, T.T., and Veríssimo, B.A. (2009). The impact of temperature on biological aspects and life table of Rhopalosiphum padi (Hemiptera: Aphididae) fed with signal grass. Florida Entomologist. 92, 569-577.
- Auad, A.M., Fonseca, M.G., Resende, T.T., and Maddalena, Í.S.C.P. (2012). Effect of Climate Change on Longevity and Reproduction of Sipha flava (Hemiptera: Aphididae). Florida Entomologist. 95, 433-444.
- Carey, F.G. and Lawson, K.D. (1973). Temperature regulation in free-swimming bluefin tuna. Comp. Biochem. Physiol.t A: Physiology, 44(2), 375–392.
- Costantini, D., Rowe, M., Butler, M.W., and McGraw, K.J. (2010). From molecules to living systems: historical and contemporary issues in oxidative stress and antioxidant ecology. Funct. Ecol. 24, 950-959.
- Filho, D.W., Torres, M.A., Tribess, T.B., Pedrosa, R.C., and Soares, C.H.L. (2001). Influence of season and pollution on the antioxidant defenses of the cichlid fish acará (Geophagus brasiliensis). Braz. J. Med. Biol. Res. 34, 719-726.
- Flynn, D.F.B., Sudderth, E.A. and Bazzaz, F.A. (2006). Effects of aphid herbivory on biomass and leaf-level physiology of Solanum dulcamara under elevated temperature and CO2. Environ. Expt. Bot. 56, 10-18.
- Galli, G.L.J., and Richards, J.G. (2012). The effect of temperature on mitochondrial respiration in permeabilized cardiac fibres from the freshwater turtle, Trachemys scripta. J. Therm. Biol. 37, 195–200.
- Goldman, K.J. (1997). Regulation of body temperature in the white shark, Carcharodon carcharias. J. Comp. Physiol. B. 167(6), 423–429.
- Halliwell, B., and Gutteridge, J.M.C. (2008). Free Radicals in Biology and Medicine. Oxford University Press, New York, USA.
- Houghton, J.T., dinGy, GriGGs, dJ, noGuer, M., vAn der linden, P.J., xiAosu d, MAskell, k, and Johnson, C.A. (2001). Climate Change 2001: The Scientific Basis. Cambridge Univ. Press, Cambridge, UK.
- Lawrence, A.J., and Soame, J.M. (2004). The effects of climate change on the reproduction of coastal invertebrates. Int. J. Avian. Sci. 146, 29-39.
- Lehninger, A.L., Nelson, D.L., and Cox, M.M. (2008). Lehninger Principles of Biochemistry (5th edition), W.H. Freeman & Co., New York, USA, pp. 707-764.
- Lenaz, G., Fato, R., Genova, M., Bergamini, C., Bianchi, C., and Biondi, A. (2006). Mitochondrial Complex I: structural and functional aspects. Biochim Biophys Acta. 1757, 1406-1420.
- Meng, L., Wong, J.H., Feldman, L.J., PGemaux, L., and Buchanan, B.B. (2010). A membrane-associated thioredoxin required for plant growth moves from cell to cell, suggestive of a role in intercellular communication. Proc. Nat. Acad. Sci. US., 107, 3900-3905.
- Nordberg, J., and Arnér, E.S. (2001). Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 31, 1287–312.
- Oliva, M., Vicente, J.J., Gravato, C., Guilhermino, L., and Galindo-Rian~o, M.D. (2012). Oxidative stress biomarkers in Senegal sole, Solea senegalensis, to assess the impact of heavy metal pollution in a Huelva estuary (SW Spain): Seasonal and spatial variation. Ecotoxicol. Environ. Saf. 75, 151–162.
- Oliveira, S.A., Auad, A.M., Souza, B., Souza, L.S., Amaral, R.L., and Silva, D.M. (2009). Tabela de esperança de vida e de fertilidade de Sipha flava (Forbes) (Hemiptera, Aphididae) alimentado com capim-elefante em diferentes temperaturas. Rev. Brasil. Entomol. 53, 614-619.
- Paital, B. (2013). Antioxidant and oxidative stress parameters in brain of Heteropneustes fossilis under air exposure condition; Role of mitochondrial electron transport chain. Ecotoxicol. Environ. Saf. 95, 69–77.
- Paital, B. (2014). Modulation of redox regulatory molecules and electron transport chain activity in muscle of air breathing fish Heteropneustes fossilis under air exposure stress. J. Comp. Physiol. B, 184, 65–76.
- Paital, B., and Chainy, G.B.N. (2010). Antioxidant defenses and oxidative stress parameters in tissues of mud crab (Scylla serrata) with reference to changing salinity. Comp. Biochem. Physiol. C, 151: 142-151.
- Paital, B., and Chainy, G.B.N. (2012). Biology and conservation of the genus Scylla in India subcontinent. J. Environ. Biol. 33, 871-879.
- Paital, B., and Chainy, G.B.N. (2014). Effects of temperature on complex I and II mediated mitochondrial respiration, ROS generation and oxidative stress status in gills of the mud crab Scylla serrata. J. Therm. Biol. 41, 104–111.
- Paital, B., and Samanta, L. (2013). A comparative study of hepatic mitochondrial oxygen consumption in four vertebrates by using Clark-type electrode. Acta Biol. Hung. 64, 152–160.
- Paital, B., Kumar, S., Farmer, R., Tripathy, N.K., and Chainy, G.B.N. (2011). In silico prediction and characterization of 3D structure and binding properties of catalase from the commercially important crab, Scylla serrata. Interdiscip. Sci. Comput. Life Sci. 3, 1913-2751.
- Paital, B., Kumar, S., Farmer, R., Tripathy, N.K., and Chainy, G.B.N. (2013). In silico prediction of 3D structure of superoxide dismutase of Scylla serrata and its binding properties with inhibitors. Interdiscip. Sci. Comput. Life Sci. 5, 69-76.
- Paital, B., Sablok, G., Kumar, S., Singh, S.K., Chainy, G.B.N. (2015). Investigating the conformational structure and potential site interactions of SOD inhibitors on Ec-SOD in marine mud crab Scylla serrata: A molecular modeling approach. Interdiscip. Sci. Comput. Life Sci. 7, 1-7.
- Passos, J.F., and Zglinicki, T.V. (2006). Oxygen free radicals in cell senescence: are they signal transducers? Free. Radic. Res. 40, 1277-83.
- Pillay, C.S., Hofmeyr, J.H., Olivier, B.G., Snoep, J.L., and Rohwer, J.M. (2009). Enzymes or redox couples? The kinetics of thioredoxin and glutaredoxin reactions in a systems biology context. Biochem. J. 417, 269–275.
- Radovanovic, T.B., Mitic, S.S.B., Perendija, B.R., Despotovic, S.G., Pavlovic, S.Z., Cakic, P.D., and Saicic, Z.S. (2010). Superoxide dismutase and catalse activities in the liver and muscle of barbel (Barbus barbus) from the Danube river, Serbia. Arch. Biol. Sci. Belgrade, 62, 97-105.
- Rhee S., Chae, H., and Kim, K. (2005). Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med., 38(12), 1543–1552.
- Rhee, S.G., Kang, S.W., Chang, T.S., Jeong, W., and Kim, K. (2001). Peroxiredoxin, a novel family of peroxidases. IUBMB Lif. 52, 35–41.
- Sanchez, W., Piccini, B., Ditche, J.M., and Porcher, J.M. (2008). Assessment of seasonal variability of biomarkers in three-spined stickleback (Gasterosteus aculeatus L.) from a low contaminated stream: Implication for environmental biomonitoring. Environ. Int. 34, 791–798.
- Takano, H., Zou, Y., Hasegawa, H., Akazawa, H., Nagai, T., and Komuro., I. (2003). Oxidative stress-induced signal transduction pathways in cardiac myocytes: involvement of ROS in heart diseases. Antioxid. Redox. Sign. 5, 789-794.
- Wohlschlag, D.E., and Juliano, R. (2003). Seasonal Changes in Bluegill Metabolism. Limnol Oceanography, 4, 195-209.
- Wollman, E.E., d'Auriol, L., Rimsky, L., Shaw, A., Jacquot, J.P., Wingfield., P. and et al. (1988). Cloning and expression of a cDNA for human thioredoxin. J. Biol. Chem., 263 15506–15512.ological Analyses. W. B. Saunders Co., Philadelphia. 357 pp.







