Keywords
Otolith dimensions, Fish length, Sea of Oman, Muscat
Introduction
The Carangidae family known popularly with their members called Jacks is among the large families of the order Perciformes and it is im-portant in both tropical and subtropical waters.
The carangid species are large, strong swim-ming, open-water and carnivorous fishes (Ran-dall 1995). In Oman, this family is composed of 48 species belonging to 24 genera (Randall 1995), and they are considered among the most important commercial commodities.
Among the three pairs of otoliths in bony fishes, sagittae have the largest pair (Harvey et al. 2000). Due to their large size and their distinct growth rings in most bony fishes, sagittae have been widely used by fisheries biologists (Boeh-lert 1985, Summerfelt and Hall 1987).
Paleontologists, oceanographers and marine biologists have used the species specific distinc-tive morphology of the sagittae and their dense structure that can resist certain degree of disinte-gration to determine the identity of fish species found in middens, sediments and stomach content of marine birds and mammals (Fitch 1964, 1969, Tripple and Beamish 1987, Ainley et al. 1981, Treacy and Crawford 1981). In the early years of the second half of the twentieth century the importance of the positive relationship between otolith size and fish size was demonstrated by Trout (1954) and Templemann and Squires (1956).
Researches in fish biology and population dy-namics get huge usage of the otolith length-total length relationship (Echeverria 1987). Further-more, the identity of the eaten fish species and their size can be estimated from their otolith re-trieved from the digestive tract of the piscivorous fishes (Aydin et al. 2004).
The relationship between fish length and oto-lith length and width has not been investigated in the fish fauna of Oman. Therefore, the aim of the present study is to find out the relationship be-tween fish length and otolith length and width in the carangid fish species C. coeruleopinnatus. These data may be used by researchers studying archaeology and food habits of piscivores to de-termine the size of fishes from the length of re-covered otolith.
Materials and Methods
Fish specimens were collected during the pe-riod March-May 2010 from waters around Mus-cat City at the Oman Sea coasts using long lines. The specimens were identified according to Ran-dall (1995). Total length (TL; most anterior point to the posterior tip of the caudal fin) was consid-ered and measured to the nearest millimeter. Sa-gittae were (total of 57 individuals, i.e., 114 oto-lith) removed through a cut in the cranium to ex-pose them, then cleaned and stored dry in glass vials and the left and right otolith were consid-ered separately. Specimens with obvious evi-dence of calcite crystallization (Strong et al. 1986) or other aberrant formations were rejected. Each sagittae, systematically placed with the sul-cus acusticus oriented through the observer and its length was determined using hand-held ver-nier callipers and defined as the longest dimen-sion between the rostrum and postrostrum axis (nomenclature of Smale et al. 1995) and width as the dimensions from the dorsal to ventral edge taken at right angles to the length through the fo-cus of the otolith. The relationship between oto-lith size (length and width) and fish length (TL) were determined using least squares linear re-gression for the following parameters: otolith length (OL)-fish length (TL) and otolith width (OW)-fish length (TL). These equations were first calculated for both left and right otoliths and ANCOVA test (Fowler and Cohen 1992) was used to check any differences between regres-sions. Similarly, the sex-linked changes in fish length and otolith length were examined statisti-cally with the ANCOVA test. The regression co-efficients were compared and when significant differences (P<0.05) were not found, the H0 hy-pothesis (bright = b left) was accepted. When the equations did not differ statistically, a single lin-ear regression was reported for each parameter (OL and OW). The significance of the linear re-gressions was verified using the ANCOVA test.
Results and Discussion
One population of C. coeruleopinnatus was used in the analysis presented in the work at hand. The linear regressions of otolith length and width against fish length for male, female and combined sexes were calculated and are given in Figures 1&2.
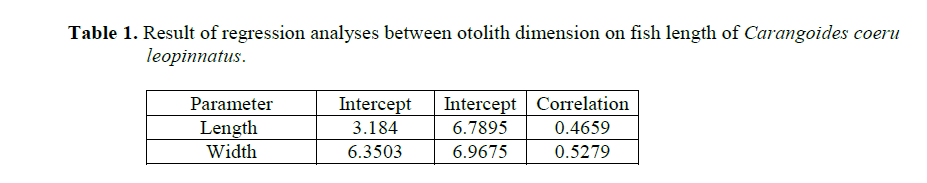
Table 1. Result of regression analyses between otolith dimension on fish length of Carangoides coeruleopinnatus.
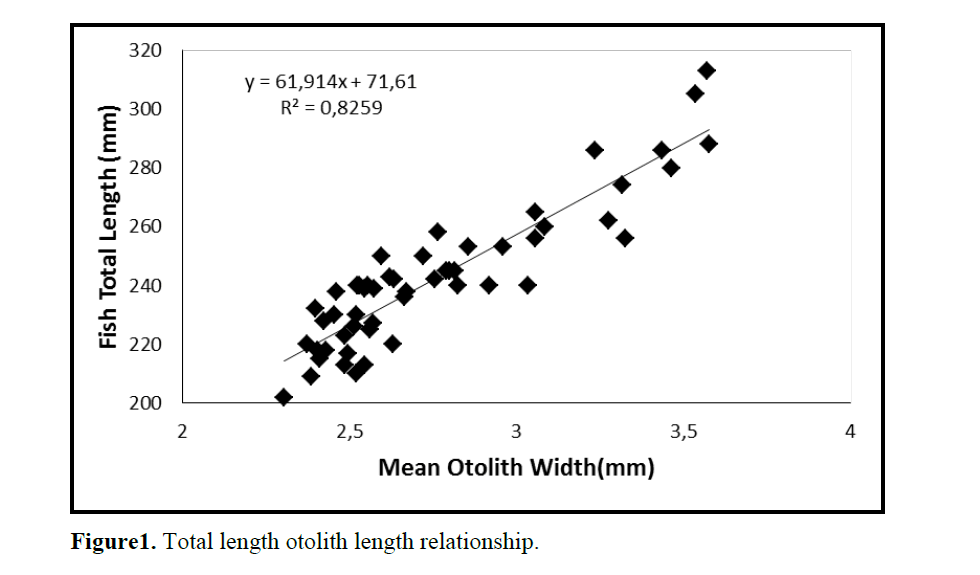
Figure1. Total length otolith length relationship.
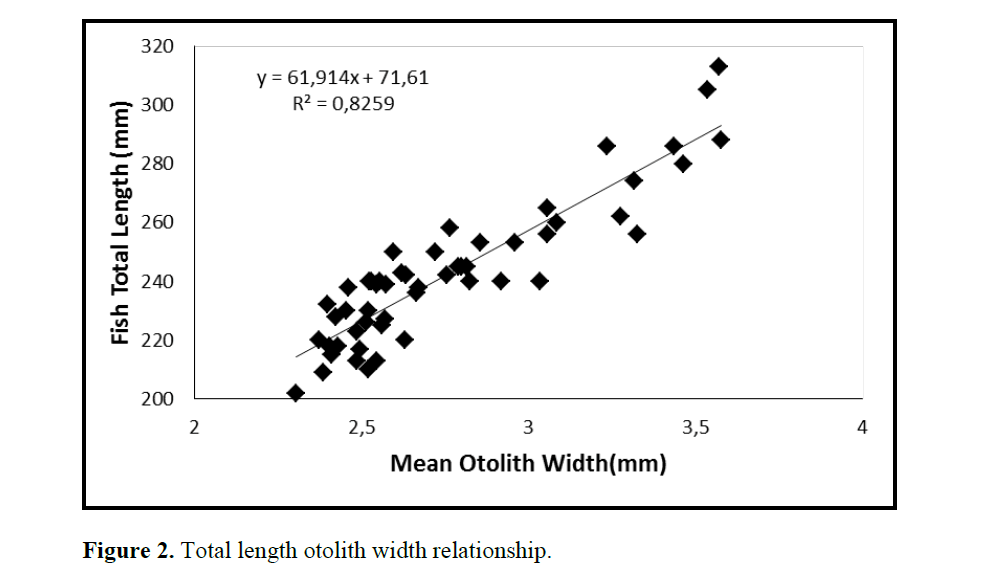
Figure 2. Total length otolith width relationship.
The result of the analyses has revealed no sta-tistical significant differences (P>0.05) between; (1) the regressions of the fish length on the left and right otolith sizes; (2) the regressions of the otolith length and width of two sexes; (3) the combined (male & female) regression of the left and right otolith length.
Among the protocols of fish identification, shape of the otolith stands as one of the main tool to separate fish species due to their high inter-specific variability (Battaglia et al. 2010). There-fore several reference works on the morphology of the fish otolith are in use specially in the field of identification of fish preys, to identify fish species depending on the shape of their otolith (Smale et al. 1995, Campana 2004, Lombarte et al. 2006, Tuset et al. 2008), however, only cer-tain geographical areas are covered and the ac-cess to reference material remains requisite (Santos et al. 2001). Thus, it is essential to esti-mate specific equations which are useful to cal-culate the size and mass of the preys.
The results of the present paper address to this need, providing TL-OL and TL-OW relationships for the carangid fish C. coeruleopinnatus. De-spite the commercial importance role of this spe-cies in the Omani waters, its biology and ecology had not been well investigated until today in Oman.
There are some limitations to the use of bio-mass reconstruction from otolith size. Several authors have shown that individuals of the same species inhabiting different areas or individuals of different stocks of the same species have dif-ferent growth rate. Such difference in the growth rate will affect the morphology of the otolith (Campana and Casselman 1993, Reichenbacher et al. 2009). The increased or reduced otolith growth rates most often are the result of changes in water temperature, water depth and diet (e.g., Lombarte and Lleonart 1993, Tuset et al. 2003, Katayama and Isshiki 2007, Mérigot et al. 2007). Elongated otolith are usually produced during increased growth rates, while more rounded oto-lith contours occur if growth is reduced (Reich-enbacher et al. 2009). Such situation was not ob-served in the results of the present study as only one population has been studied.
The effect of chemical and mechanical abra-sion in the digestive track of predators on the otolith traits might become factor to underesti-mate otolith size (Jobling and Breiby 1986, Granadeiro and Silva 2000). Similar results were obtained for some otolith of the species in ques-tion retrieved from the stomach of fishes col-lected from Sea of Oman. In these otoliths, the ornaments usually found on the mesial side of the otolith have been severely abrassed and the ante-rior and posterior edges of the otolith have been deteriorated and the size of the otolith looks much smaller than that of the otolith recovered directly from the fish body of the same species (Sulaiman et al. 2007, Saad 2005, Khalid 2007).
Unlike the previous studies that focused on the relationship between fish and sagitta sizes (Wyllie 1987, Gamboa 1991, Granadeiro, Silva 2000, Harvey et al. 2000, Waessle et al. 2003, Battaglia et al. 2010), this paper supplies addi-tional information by considering both the otolith length (OL) and otolith width (OW). It is more suitable to calculate more than one equation (TL-OL and TL-OW) since the tip of the otolith ros-trum or the dorsal or ventral edges of the otolith may be damaged, making it impossible to meas-ure the OL or OW.
In contrast with the findings of Harvey et al. (2000) and Waessle et al. (2003) the otolith of the species in question did not show significant dif-ferences in size between left and right sagittae. This finding is in agreement with the results of Battaglia et al. (2010).
In spite of all data fitted well with the linear regressions obtained in the present study, it is ad-visable to use these equations within the fish size range limit reported for this species in the results section. Authors who studied wide range of fish length and include larvae in their sample, have supplied two different TL- OL regressions, one for the small sized fish and another for the adult specimens (Nishimura and Yamada 1988, Lin-kowski 1991). Such situation is not applicable to the results obtained in the present study as no lar-vae were present in the sample as the individuals of C. coeruleopinnatus collected in the present paper belong to the 202- 313 mm TL range, the regressions TL-OL and TL- OW calculated here in can be accepted.
Conclusions
There are no differences in length and width of the right and left otolith of fish specimens ob-tained. This is also true for the regressions be-tween fish length on the length and width of oto-lith, so a single linear regression was plotted against total length (TL) for otolith length (OL) and otolith width (OW). These relationships pro-vide a reliable tool in feeding studies and also provide support to palaeontologists in their re-search on fish fossils.
Acknowledgements
Our sincere thank goes to the Ministry of Ag-riculture and Fisheries Wealth, Marine Science and Fisheries Centre and the directorate of Agri-culture and Fisheries Developmental Fund for giving us the opportunity to work on the fish samples within the qualitative and quantitative distribution of marine organisms in Sultanate of Oman and to provide the appropriate financial support.
606
References
- Ainley, D. G., Anderson, D.W., Kelly, P. R., (1981). Feeding ecology of marine cormo-rants in southwestern North America, Con-dor, 83: 120-131.ndoi: 10.2307/1367418
- Aydin, R., Calta, M., Sen, D., Coban, M. Z., (2004). Relationships between fish lengths and otolith length in the population of Chondrostoma regium (Heckel, 1843) in-habiting Keban Dam Lake, Pakistan Journal of Biological Sciences, 7: 1550-1553.ndoi: 10.3923/pjbs.2004.1550.1553
- Battaglia, P., Malara, D., Romeo, T., Aadaloro, F., (2010). Relationship between otolith size and fish size in some mesopelagic and bath-ypelagic species from the Mediterranean Sea (Strait of Messina), Scientia Marina, 74: 605-612.ndoi: 10.3989/scimar.2010.74n3605
- Boehlert, G. W., (1985). Using objective criteria and multiple regression models for age de-termination in fishes, Fishery Bulletin, 83: 103-117.
- Campana, S. E., Casselman, J. M., (1993). Stock discrimination using otolith shape analysis, Canadian Journal of Fisheries and Aquatic Sciences, 50: 1062-1083.ndoi: 10.1139/f93-123
- Campana, S. E., (2004). Photographic atlas of fish otoliths of the Northwest Atlantic Ocean, pp. 284.Ottawa, Ontario: NRC Re-search Press.
- Eecheverria, T. W., (1987). Relationship of oto-lith length to total length in rockfishes from northern and central California, Fishery Bulletin, 85: 383-387.
- Fitch, J. E., (1964). The fish fauna of the Playa del Rey Locality, a southern California ma-rine Pleistocene deposit, Los Angeles City Museum of Contemporary Science, 82: 3-35.
- Fitch, J. E., (1969). Fossil records of certain schooling fishes of the California current system, CALCOFI Report, 13: 71-80.
- Fowler, J., Cohen, L., (1992). Practical statistics for field biology. John Wiley and Sons, Chichester, New York, Brisbane, Toronto, 227pp.
- Gamboa, D. A., (1991). Otolith size versus weight and body-length relationships for eleven fish species of Baja California, Mex-ico, Fishery Bulletin, 89: 701-706.
- Granadeiro, J. P., Silva, M. A., (2000). The use of otoliths and vertebrae in the identification and size-estimation of fish in predator-prey studies, Cybium, 24: 383-393.
- Harvey, J. T., Loughlin, T. R., Perez, M. A., Ox-man, D. S., (2000). Relationship between fish size and otolith length for 63 species of fishes from the eastern North Pacific Ocean. NOAA Technical Report NMFS, 150: pp. 35.
- Jobling, M., Breiby, A., (1986). The use and abuse of fish otoliths in studies of feeding habits of marine piscivores, Sarsia, 71: 265-274.
- Katayama, S., Isshiki, T., (2007). Variation in otolith macrostructure of Japanese flounder (Paralichthys olivaceus): a method to dis-criminate between wild and released fish, Journal of Sea Research, 57:180ÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâââÂÂìÃÂ
âÂÂ186.ndoi: 10.1016/j.seares.2006.09.006
- Khalid, M. H., (2007). Food items of some pis-civorous fishes obtained from the Sea of Oman. Quarterly Report, Ministry of Envi-ronment, Iran 345pp.
- Linkowski, T. B., (1991). Otolith microstructure and growth patterns during the early life history of lanternfishes (family Myctophi-dae), Canadian Journal of Zoology, 69: 1777-1792.ndoi: 10.1139/z91-247
- Lombarte, A., Lleonardti, J., (1993). Otolith size changes related with body growth, habitat depth and body temperature, Environmental Biology of Fishes, 37: 297ÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâââÂÂìÃÂ
âÂÂ306.ndoi: 10.1007/BF00004637
- Lombarte, A., Chico, ÃÂÃÂÃÂâÂÂÃÂâ â≢ÃÂÃÂââ¬Ã
¡ÃÂâââÂÂìââÂÂâ., Parisi-Baradad, V., Olivellaii, R., Piera, J., Garcia-Ladona, E., (2006). A web-based environment from shape analysis of fish otoliths. The AFORO database (https://www.cmima.csic.es/aforo/), Scientia Marina, 70: 147-152.
- MÃÂÃÂÃÂâÂÂÃÂâ â≢ÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂérigot, B., Letourneur, Y., Lecomte-Finiger, R., (2007). Characterization of localpopulations of the common sole Solea solea (Pisces: Soleidae) in the NW Mediterranean through otolith morphometrics and shape analysis, Marine Biology, 151: 997-1008.ndoi: 10.1007/s00227-006-0549-0
- Nishimura, A., Yamada, J., (1988). Geographical differences in early growth of walleye Pollock Theragra chalcogramma, estimated by back-calculation of otolith daily growth increments, Marine Biology, 97: 459-465.ndoi: 10.1007/BF00391041
- Randall, J. E., (1995). Coastal fishes of Oman. Bathurst, Australia: Crawford House Publishing Pty Ltd. 439pp.
- Reichenbacher, B., Kamrani, E., Esmaeili, H. R., Teimori, A., (2009). The endangered cyprinodont Aphanius ginaonis (Holly, 1929) from southern Iran is a valid species: evidence from otolith morphology, Environmental Biology of Fishes, 86: 504-521.
- Saad, S. Y., (2005). On the food items of some piscivorous fishes collected from the Sea of Oman, Annual Report, Ministry of Environment, 456pp.
- Santos, M. B., Clarkeii, M. R., Pierce, G. J., (2001). Assessing the importance of cephalopods in the diets of marine mammals and other top predators: problems and solutions, Fisheries Research, 2: 121-129.ndoi: 10.1016/S0165-7836(01)00236-3
- Strong, M. B., Neilson, J., Hunt, J., (1986). Aberrant crystallization of Pollock otoliths, Canadian Journal of Fisheries and Aquatic Sciences, 43: 1457-1463.ndoi: 10.1139/f86-180nSulaiman, D. F., Hameedi, N. B., Ilahii, U. K., (2007). Food and feeding habits of some piscivorous fishes collected from the Sea of Oman. Scientific Report No. 5, Ministry of Fisheries, Iran, 98pp.
- Summerfelt, R.C., Hall, G. E., (Eds.) (1987). The age and growth of fish. Iowa State University Press, Ames, 544p.
- Templemann, W., Squires, H. J., (1956). Relationship of otolith lengths and weights in the haddock, Melanogrammus aeglefinus (L.), to the growth of the fish, Journal of Fisheries Research Board of Canada, 13: 467-487.ndoi: 10.1139/f56-029
- Treacy, S. D., Crawford, T.W., (1981). Retrieval of otoliths and statoliths from gastrointestinal contents and scats of marine mammals, Journal of Wildlife Management, 45: 990-993.ndoi: 10.2307/3808110
- Tripple, E. A., Beamish, F.W. H., (1987). Characterizing piscivory from ingested remains, Transaction of the American Fisheries Society, 116: 773-776.ndoi: 10.1577/1548-8659(1987)116<773:CPFIR>2.0.CO;2
- Trout, G. C., (1954). Otolith growth of the Barents Sea cod. Rapp P -V RÃÂÃÂÃÂâÂÂÃÂâ â≢ÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂéun Cons Int Explor Mer, 150: 297-299.
- Tuset, V. M., Lombarte, A., Assis, C. A., (2008). Otolith atlas for the western Mediterranean, north and central eastern Atlantic, Scientia Marina, 72S1: 7-198.
- Waessle, J. A., Lasta, C. A., Bavero, M., (2003). Otolith morphology and body size relationships for juvenile Sciaenidae in the RÃÂÃÂÃÂâÂÂÃÂâ â≢ÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂÃÂo de la Plata estuary (35-36ÃÂÃÂÃÂâÂÂÃÂâââÂÂìÃÂ
áÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂðS), Scientia Marina, 67: 233-240.
- Wyllie, E. T., (1987). Relationship of otolith length to total length in rockfishes from northern and central California, Fishery Bulletin, 85: 383-387.









