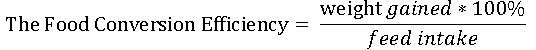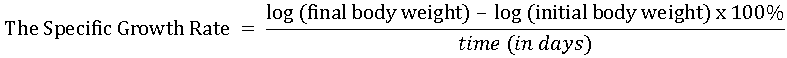Keywords
Yasama, Ölüm, Örnekler, Yavru, Tank, Sistem
Introduction
Mullets (Mugilidae) are common fishes in the coastal waters of tropical and subtropical countries of the world. Sixteen species have so far been identified in West Africa (Fowler, 1936; Cadenat, 1954 and Blay, 1995), and these constitute an im-portant proportion of the catches of commercial and subsistence fishermen in some countries in this area (Brulhet, 1975; Payne, 1976). The stripped mullet, Mugil cephalus, is perhaps the most widespread and abundant inshore teleost (Collins, 1985). Size is generally more biologi-cally relevant than age in fishes, due to several ecological and physiological factors that are more size-dependent than age dependent. Variability in size has important implications for diverse aspects of fisheries science and population dynamics (Er-zini, 1994).
Adult M. cephalus are highly euryhaline, and survive in a range of salinities from 0o/oo in fresh water to hypersaline waters. Adult M. cephalus can be found along the open coasts, but juveniles are most likely found in estuaries (Collins, 1985). Silva and De Silva (1981) reported that the per-centage of grey mullet catches increased with in-creasing salinity. Larger fishes were found in the deeper areas. Stability of the water column and suitable food in coastal lagoons, river deltas, and estuarine mangrove areas have been identified as important factors influencing the recruitment of juvenile Mugilidae (Blaber and Blaber, 1980; Bla-ber, 1987; Vieira, 1991)
In spite of the abundant biological data on this species from other countries, comparative data from brackish water lagoons in Africa, with Nige-ria in particular is highly insufficient. Yet the fish constitutes a mainstay of the fisheries resources in the coastal communities; if the potentials are well exploited, a viable mullet fishery and culture can be maximally sustained. In this report, the salinity tolerance of grey mullet, Mugil cephalus fry in La-gos Lagoon (high brackish water), Nigeria, are in-vestigated in the laboratory to give information for culture of the species in brackish and fresh waters.
Materials and Methods
Collection of specimens
Collection of live fry from the Lagos Lagoon was made using hand nets at shallow portions of the lagoon near the shore for the salinity tolerance experiments.
Experimental set-up
The experiment was carried out in translucent rectangular white plastic tanks each of 35 litres ca-pacity and filled with 25 litres of water. Replicate tanks for each salinity regime of fresh water (0‰), 5‰, 10‰, 15‰, 20‰, 25‰ and sea water (30‰) were used. The salinity in each tank was deter-mined using a refractometer. The freshwater was obtained from tap-water that has been dechlorin-ated for over 24 hours, while the sea water was ob-tained from the Lagos bar-beach. The experi-mental tanks were placed in a compartment where there was a reduced effect of direct sunlight. The fries of M. cephalus used were acclimatized in la-goon water of 15‰ salinity for five days. Ten live specimens each were then placed in the experi-mental tanks of varied salinities after the acclima-tization period. They were fed with formulated coppens feed at a daily ration of 5% of their total body weight. Adjustments in weight of feed fed were done fortnightly after measuring the weight of fish remaining in tanks. Growth, survival and mortality of the specimens were monitored over eight weeks. Change of water in the tanks and measurements were done forthnightly. Aerating air-pumps were used during the experimental pe-riod to aerate the tanks.
Physical – chemical parameter measurement
The physico-chemical parameters were deter-mined twice a week. Water temperature was meas-ured using a simple mercury-in-glass thermome-ter. The salinity of the water was determined us-ing a Refractometer (BIOMARINE, Aqua Fauna Model. Dissolved oxygen (DO) of the water sam-ples was determined using a Jenway DO Meter (Model 4310). The pH values were determined us-ing a Jenway Hanna pH meter (HI 991301 Model).
Length – weight measurement
The total length (TL) of the specimens was measured on a measuring board to the nearest 0.1 centimeter. The total weight of the fish was taken on a ‘Sartorious’ top loading balance (Model 1106 2608053) or a triple beam balance to the nearest tenth of a gram.
Statistical analyses
The data obtained were subjected to statistical analyses using different formulae:

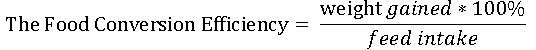
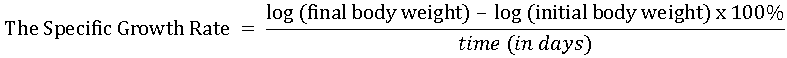
To test if the differences in mortality observed in the different salinity levels were significant, ANOVA was employed.
Results and Discussion
Survival / Mortality of M. cephalus fry in var-ied salinities
Salinity tolerance experiment carried out showed that the fries of M. cephalus could survive in varying salinity range of 5‰ to 25‰. The sum-mary of the weekly survival / mortality record for M. cephalus fry in the tanks is given in Table 1 while Figure 1 showed the percentage survival curve for M. cephalus in varied salinity regimes. The ANOVA test indicated that there was no sig-nificant variation (p > 0.05) in the differences ob-served in mortality in the various salinity regimes.
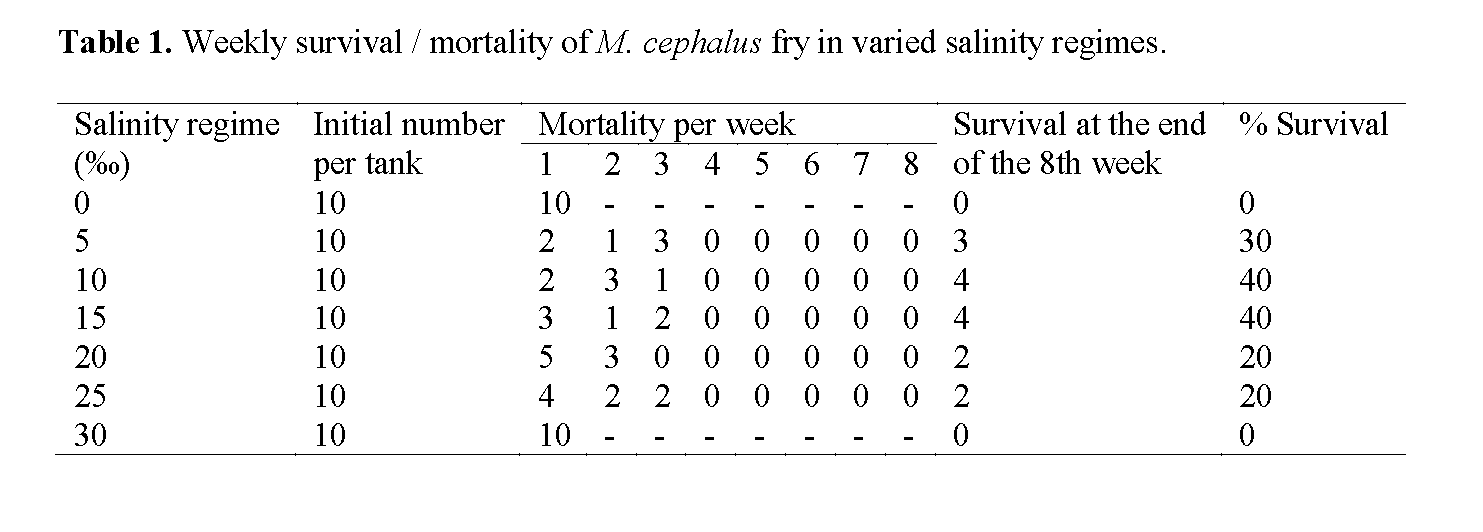
Table 1: Weekly survival / mortality of M. cephalus fry in varied salinity regimes.
Physico-chemical parameters in culture tanks
The weekly illustration of the physico-chemi-cal parameters during the salinity tolerance exper-iment is presented in Figure 2. The pH ranged from 7.60 – 10.15 (mean 7.89±0.13); DO ranged from 2.47 – 10.16 (mean 7.08±0.46) and temperature ranged from 28.6 – 30.0oC (mean 29.02±0.13).
Effects of salinity on the growth of M. cephalus
Summary of the percentage gain or loss in total length and body weight of M. cephalus in varied salinity levels is presented in Table 2. The speci-mens in 0‰ and 30‰ salinity regimes all died within the 1st week of the experiment. The initial mean total length of the fries ranged from 1.9-2.2 cm (2.04 ±0.10); while the initial mean body weight was 0.10g (0.1 ±0.00). The final mean total length was 3.0 ±2.06, while the final mean body weight was 0.69 ±0.50. The highest % gain in mean length of 22.02 was recorded in the 15‰ and 20‰ salinity regimes, while the lowest value of 17.43 was in the 5‰ salinity regime. The highest % gain in mean weight of 22.99 was recorded in the 15‰ and 20‰ salinity regimes, while the lowest value of 11.49 was in the 5‰ salinity re-gime.
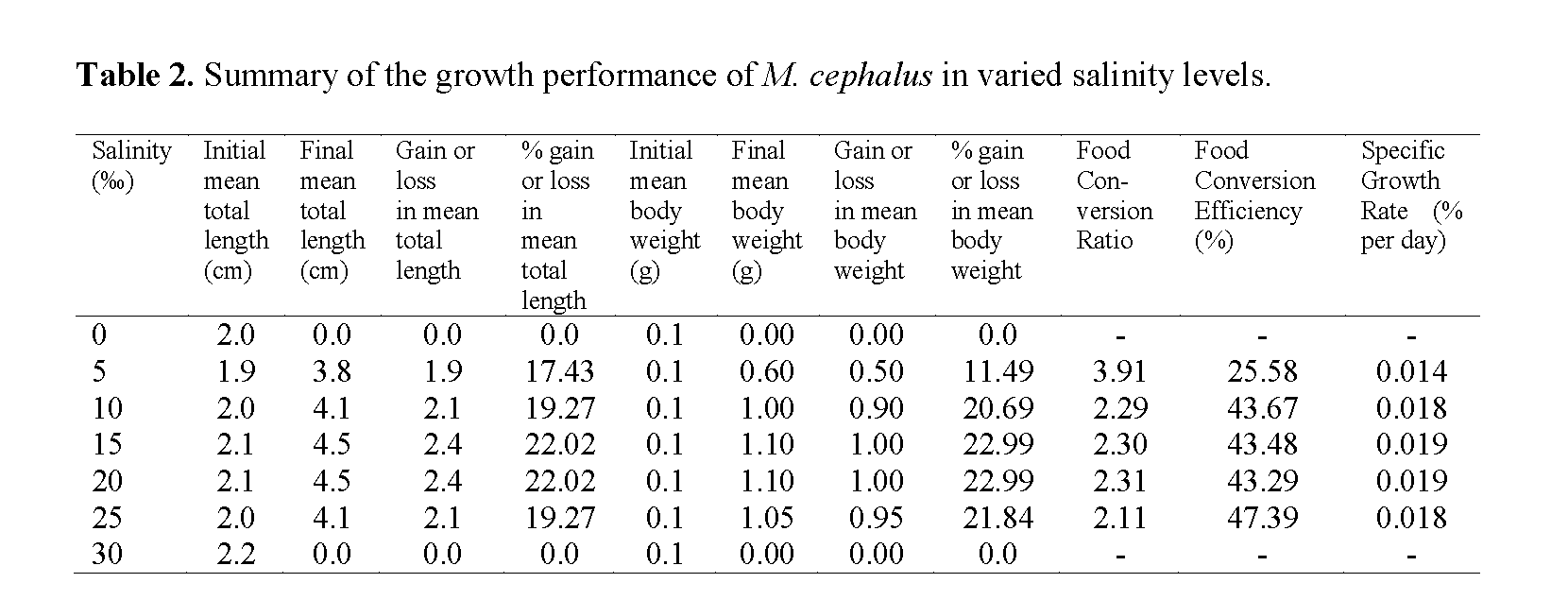
Table 2: Summary of the growth performance of M. cephalus in varied salinity levels.
The FCR values of the fish in all the regimes ranged from 2.11-3.91, with the highest value recorded in the 5‰ regime and the lowest in the 25‰ regime. The FCE (%) values ranged from 25.58-47.39, with the highest value recorded in the 25‰ regime and the lowest in the 5‰ regime. The SGR values of the fish in all the salinity regimes ranged from 0.014-0.019, with the highest value recorded in the 15‰ and 20‰ regimes, while the lowest was recorded in the 5‰ regime
The differences in growth between different sa-linity regimes examined in the present study were not found to be significant and this agreed with the report by McDonough and Wenner (2003). Car-dona (2000) revealed that the metabolic rate of young specimens was negatively affected by high salinity levels and that an improved growth perfor-mance was achieved in freshwater and oligohaline water (0.1-5 ‰). A stratified study on microhab-itat use carried out on the Island of Minorca (Bal-earic archipelago), western Mediterranean sea, demonstrated that juvenile specimens, shorter than 200mm (TL), concentrated all year round in fresh-water or oligohaline sites. Mesohaline areas were usually avoided, except in summer. Immature fish, with a total length between 201 and 300mm showed a similar pattern although in some seasons avoided freshwater sites. The habitat selection pattern of adults, i.e. fish longer than 301mm, changed seasonally due to their offshore migration during the spawning season (from late summer to early winter). They usually showed a greater pref-erence for polyhaline areas and strongly avoided freshwater sites, which might also be due to their shallowness (Cardona, 2000).
The differences in growth or gain in length and weight between different salinity regimes exam-ined in this study were not found to be significant. The high survival rate of M. cephalus fry (1.5-2.0 cm TL) in water of different salinities (5-25‰) demonstrated in this present investigation revealed the ability of grey mullets to adapt to wide salinity fluctuations. The mortality experi-enced within the 1st week in the 0‰ and 30‰ sa-linities was probably connected with the abrupt transfers. Ciccoti et al (1994) noticed a high sur-vival rate and tissue osmolality regulation of M. cephalus juveniles (SL = mean 28.05, SD = 3.54mm) acclimated to freshwater gradually in 48 hours. The morphological and biochemical as-pects of esophagus and gills in juveniles are simi-lar to those of the adult, suggesting that osmotic regulatory mechanisms are precociously devel-oped to allow the colonization of eutrophic inland waters. From their investigation, Hotos and Vlahos (1998) highlighted that M. cephalus fry ac-climated in aquaria with 20‰ sea water accli-mated gradually over 35-40 days with no signs of stress. By direct transfer from 20‰ to salinities of 35, 40, 45, 50, 55, 60, 65, 70, 75 and 80‰, mor-tality occurred at salinities greater than 45‰.
The physical and chemical parameters ob-tained in the present study showed a similar pat-tern in all the varied salinity regimes. There were no wide fluctuations in those parameters and is clear that M. cephalus can thrive well under a trophic climatic condition.
Bulli and Kulikova (2006) reported the re-sponse of early juveniles of the harder, Liza haem-atocheila (= Mugil soiuy) to changes in water sa-linity, and the growth and survival of larvae in wa-ter of different salinity levels. They discovered that at decreasing salinity, the growth rate, the content of defatted dry matter, and the content of lipids increased. In freshwater, the stock lipids (triacylglycerols) accumulate more intensively.
From the FCR and FCE values, it could be seen that the fry in the 25‰ salinity regime had the lowest FCR, hence the best FCE, which meant that there was more effective utilization of less unit weight of feed to produce a unit weight of flesh than in the other regimes. However, the best SGR was in the 15‰ and 20‰ salinity regimes, which were slightly higher than those in 10‰ and 25‰ regimes.
Conclusions
The ability of grey mullet fry to tolerate a wide range of salinity regime demonstrated in this study further agreed to the euryhalinity of the species and its potential candidature for brackish water fish culture.
Acknowledgments
The authors express their profound gratitude to Professor (Emeritus) Kola Kusemiju for his inval-uable contributions to the conception and design of the experiment; and the revising of the draft of the manuscript for intellectual content. We thank Mr. Anipole Biodun for the editorial assistance.
419
References
- nBlaber, S.J.M., (1987). Factor influencing recruit-ment and survival of mugilids in estuaries and coastal waters of Southeastern Africa, Amer-ican Fisheries Society Symposium, 1: 507–518
- nBlaber, S.J.M., Blaber, T.G., (1980). Factors af-fecting the distribution of juvenile estuarine and inshore fish, Journal of Fish Biology, 17: 143-162. doi: 10.1111/j.1095-8649.1980.tb02749.x
- nBlay, J., (1995). Food and feeding habits of four species of juvenile mullet (Mugilidae) in a tidal lagoon in Ghana, Journal of Fish Biol-ogy, 46: 134-141. doi: 10.1111/j.1095-8649.1995.tb05952.x
- nBrulhet, J., (1975). Observations on the biology of Mugil cephalus ashanteensis and the possi-bility of its aquaculture in the Mauritanean coast, Aquaculture, 5: 271-281. doi: 10.1016/0044-8486(75)90004-6
- nBulli, L.I., Kulikova, N.I., (2006). Adaptive ca-pacity of the larvae of the haarder Liza haem-atocheila (Mugilidae, Mugiliformes) under decreasing salinity of the environment, Jour-nal of Ichthyology, 46(4): 525-535
- nCadenat, J., (1954). Note d’ichthyologie oust-afri-cain 8. Regime alimentaire sur les mullets de la cote occidental d’Afrique, Bulletin de I’In-stitute francais d’Afrique noire (ser. A) 16: 584-591
- nCardona, L., (2000). Effects of salinity on the hab-itat selection and growth performance of Mediterranean flathead grey mullet, Mugil cephalus (Osteichthyes, Mugilidae), Estua-rine, Coastal and Shelf Science, 50(5): 727-737. doi: 10.1006/ecss.1999.0594
- nCiccotti, E., Marino, G., Pucci, P., Cataldi, E., Cataudella, S., (1994). Acclimation trial of Mugil cephalus juveniles to freshwater: mor-phological and biochemical aspects, Environ-mental Biology of Fishes, 43(2): 163-170. doi: 10.1007/BF00002487
- nCollins, M.R., (1985). Species profiles: Life histo-ries and environmental requirements of coastal fishes and invertebrates (South Flor-ida) – striped mullet. U.S. Army Corps of En-gineers, TR EL – 82.4 11pp
- nErzini, K., (1994). An empirical study of variabil-ity in length and age of marine fishes, Journal of Applied Ichthyology, 10: 17-41. doi: 10.1111/j.1439-0426.1994.tb00140.x
- nFowler, H.W., (1936). The marine fishes of West Africa. Bulletin of the American Museum of Natural History, 70: 1-605
- nHotos, G.N.,Vlahos, N., (1998). Salinity tolerance of Mugil cephalus and Chelon labrosus (Pi-sces: Mugilidae) fry in experimental condi-tions, Aquaculture, 167(3-4): 329-338. doi: 10.1016/S0044-8486(98)00314-7
- nMcDonough, C.J., Wenner, C.A., (2003). Growth, recruitment and abundance of juvenile Mugil cephalus in South Carolina estuaries, Fisher-ies Bulletin, 101: 343-357
- nPayne, A.L., (1976). The relative abundance and feeding habits of the grey mullet species oc-curring in an estuary in Sierra Leone, West Africa, Marine Biology, 35: 277-286. doi: 10.1007/BF00396875
- nSilva, E.I., De Silva, S.S., (1981). Aspects of the biology of grey mullet, Mugil cephalus L., adult populations of a coastal lagoon in Sri Lanka, Journal of Fish Biology, 19(1): 1-10. doi: 10.1111/j.1095-8649.1981.tb05806.x
- nVieira, J.P., (1991). Juvenile mullets (Pisces: Mu-gilidae) in the estuary Lagoa dos Patos, RS, Brazil, Copeia, 409-418. doi: 10.2307/1446590