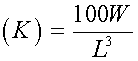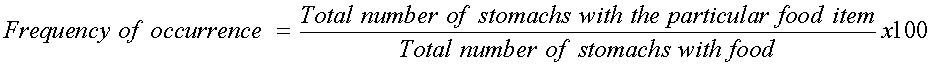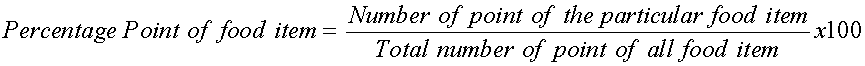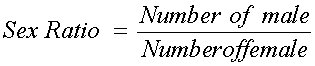Keywords
African Carp; River Benue; Length-Weight Relationship; Food and Feeding Habit
Introduction
In sub Saharan Africa, fishing activity is usually not monitored leading to the excessive use of obnoxious fishing practice and deliberate disposal of toxic and hazardous chemicals into water bodies. This has resulted in significant loss of aquatic life, habitats destruction and generally reduction in fish yield (Adeyemo, 2004, Solomon et al., 2012). Currently many fish resources in Africa are depleted; inadequate management and unscrupulous practices is threatening aquatic life and natural habitats (Adeyemo, 2004), there is an urgent need to take appropriate measures to mitigate continuous loss of aquatic lives. According to Umaru et al., (2015), domestication and culture of commercially important fish species remains the key to mitigating further decline and possible extinction of these fishes. One of the important aspects of fish that needs to be focused for a successful domestication program includes aspect of the biology of the fish. Okafor et al (2012) rightly opined that most commercially important fish species have not been successful cultured on a commercial scale due to insufficient knowledge of the biology of these fishes, hence, in order to cultivate any of these fish species successfully in captivity, a good knowledge of their biology is important.
Studies on fish biology are indispensible aspect of sustainable management and conservation of fish biodiversity (Solomon et al., 2012). One of the most commonly estimated biological aspects of fish is the length-weight relationship and condition factor (Mendes et al., 2004). According to Gulland, (1983), Sumbuloglu and Sumbuloglu (2000) and King, (1996), length-weight relationship is an important factor in biological study and their stock assessments of fishes as it literarily describes the functional regime in weight distribution per unit size of sub-population. Condition factor is also important for assessing the relative wellbeing of the fish population (Kulbicki et al. 1993 and King 1996). Adeyemi (2010) further opined that length-weight relationships can be used to assess growth fluctuations due to changes in food composition, environmental variables, and spawning conditions among other factor. Another aspect of fish biology that needs to be studied for a successful domestication program is the feeding habit of the fish. According to Wootton (1992), food and feeding habits are indispensable part of biological and taxonomic studies, because it is an essential function of an organism as growth, development and reproduction are all-dependent on energy that enters an organism in the form of food. Pius and Benedicta (2002) further stated that the assessment of the stomach content of a fish reduce intra and inter specific competition for ecological niche as it is vital in providing straight forward models of stomach content and feeding dynamics. Therefore, it is important to understand the spectrum of food preferred by the fish in the wild so as to adopt same in captivity.
Labeo is one of the most common genus in the family Cyprinidae found in major rivers of African countries such as Nigeria, Senegal, Gambia, Ivory Coast, Liberia, Zaire, and Gabon (Ayotunde et al; 2007). Four species of this genus are largely found in rivers and they includes, Labeo senegalensis, Labeo pseudocoubie, Labeo rhohita, and Labeo coubie, of these four species recorded in Africa, Labeo coubie is about the most common and can grow to about 700mm in length and 10kg in weight (Idodo-umeh, 2005, Ayotunde et al., 2007). They are highly valued fish food in African countries and usually known for their sweet tastes and rich source of protein (Rahman, 1989, Ayotunde et al., 2007). In a bid to conserve wild stock of two importantly exploited species of this genus in River Benue (Labeo coubie and Labeo senegalensis), this study was designed to investigate some aspects of the biology of those fishes so as to provide background information for possible domestication of the fishes.
Materials and Methods
Description of Study Area
The study was conducted at the lower River Benue axis in Makurdi the capital of Benue State in Nigeria, it is located at Longitude 7°43°N and Latitude 8°32°E (Figure 1). The town is divided into the North and the South bank by the River Benue. The river contains several species of fish which are of high economic importance to the people of Benue State, notable among these species is the Labeo coubie and Labeo senegalensis, hence their choice for this study.
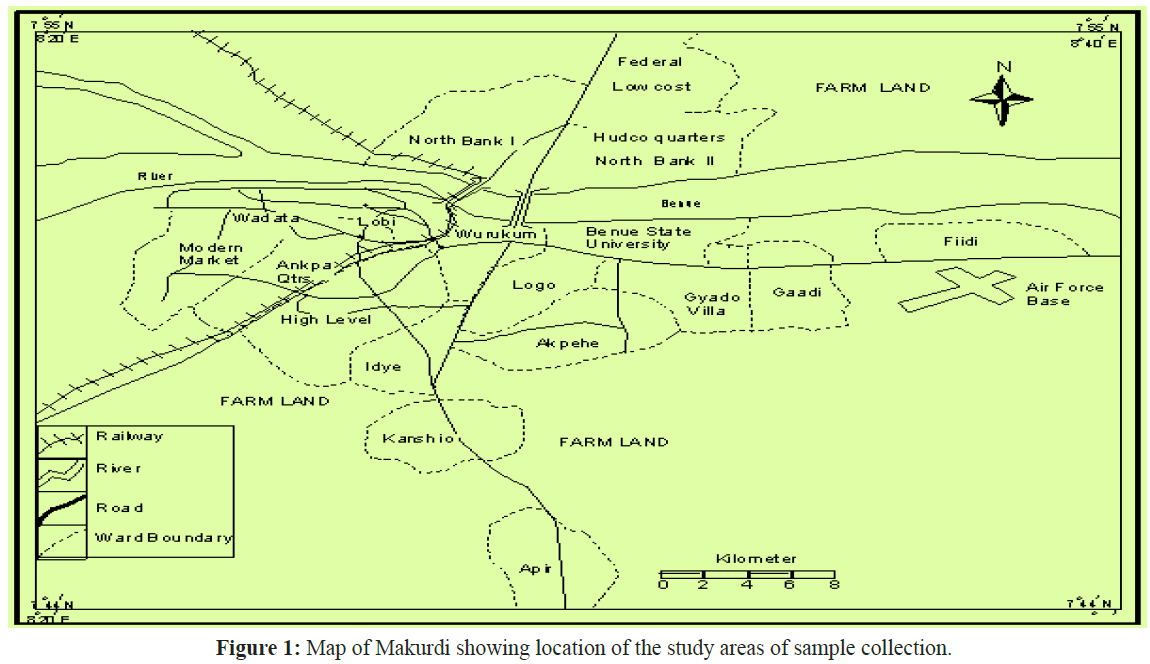
Figure 1: Map of Makurdi showing location of the study areas of sample collection.
Fish sample Collection
Fish sample for this study were obtained from fishermen at three major landing sites in Makurdi. The fishing gears used by the fishermen in catching the fish includes; traps, seine nets, cast net, gill nets, clap nets, hooks and line, while crafts were basically canoe and calabash. Target species for the study (Labeo coubie and Labeo senegalensis) were randomly sampled at each site every fortnight over a period of four months (June 2013 – September 2013 at this time fish abundance is high). Sampling time was between 6:00am and 8:00am; this is about the time fishermen would be returning to landing site after fishing through the night. Collected samples were fixed in ice chest and moved to the Department of Fisheries and Aquaculture University of Agriculture Makurdi for data collection of biometric, sexing and observation of the stomach content.
Length- Weight Measurement
A total of 100 fish specimens each of Labeo coubie and Labeo senegalensis were obtained from fishermen at the fish landing sites. The total and standard lengths of each fish species were measured in centimeters (cm) using a meter rule, while the weight were taken in grams (g) using an electronic weighing balance.
The length-weight relationship was calculated using the equation below;
LogW = log a + b log L
The condition factor (K) was calculated according to the equation by Pauly (1983) below:
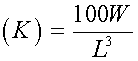
Stomach Analysis
The ventral parts of the fishes were dissected and the stomachs immediately preserved in sterile bottles containing 5% formalin. Individual stomach contents were emptied into separate petri-dishes. While some stomach contents were identified macroscopically, others were identified microscopically using a light microscope. The component food items were identified using identification guide provided in the laboratory of the Department of Fisheries and Aquaculture University of Agriculture Makurdi. The food items encountered were analyzed using frequency of occurrence method (Hynes, 1950) and point method (Cortes, 1997) as stated in the formulae below.
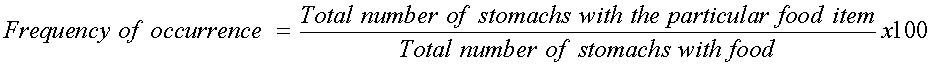
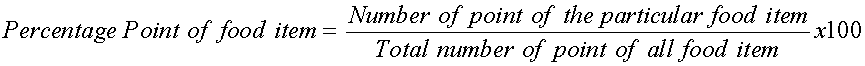
Percentages gotten from these two methods were pulled together and the means and standard error determined
Determination of Sex Ratio
The fishes of the various species were separated into male and female using specific features that were peculiar to the different sexes. These features include shape of the reproductive organ and presence of nubs on the abdominal segment. Sex ratio of each species was then determined by counting the number of male and female specimens of each species sampled in the period of study and expressed as ratio as stated below,
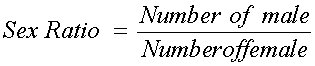
Statistical Analysis
The data analysis was carried out using Minitab 14 software. Biometric parameters were subjected to Analysis of Variance to determine if significant differences exist between the different sexes of the two species, means were then separated by Fisher’s least significant difference. Test for goodness of fit was determined statistically using Chi- square (x2) test on sex ratio.
Results
The biometric parameters measured in this study are shown in Table 1; total length of male and female Labeo coubie ranges from 14.45-39.50cm respectively while those of L. senegalensis ranged from 13.90 to 39.70. Mean values recorded were higher in males (26.78 and 23.53 for L. coubie and L. senegalensis respectively) and lowest in female (24.36 and 22.25 for L. coubie and L. senegalensis respectively). The standard length recorded in all the fish sampled ranges from 13.90-44.10cm, mean standard length was however higher in male L. coubie (29.58) and lowest in female of the same species (21.97). Weight was significantly higher in male L. senegalensis (334.15) and lowest in female L. coubie (215.6).
| |
Total length |
Standard length |
Weight |
| |
Min. |
Max. |
Mean |
Min. |
Max. |
Mean |
Min. |
Max. |
Mean |
| L. coubie Male |
17.80 |
39.50 |
26.78 ± 6.41a |
18.60 |
44.10 |
29.58 ± 7.70a |
99.50 |
913.00 |
324.61 ± 4.33b |
| L. coubie Female |
14.45 |
36.25 |
24.36 ± 6.91b |
13.90 |
33.70 |
21.97 ± 5.79d |
88.50 |
801.00 |
215.6 ± 2.69d |
| L. senegalensis Male |
13.90 |
39.70 |
23.53 ± 7.27c |
18.10 |
43.50 |
28.22 ± 7.85b |
90.50 |
973.00 |
334.15 ± 3.69a |
| L. senegalensis Female |
13.90 |
36.00 |
22.25 ± 6.29d |
18.50 |
42.40 |
27.33 ± 7.21c |
101.50 |
981.90 |
312.08 ± 3.41c |
Table 1: Length and weight distribution of Labeo coubie and Labeo senegalensis from lower River Benue.
The length-weight relationship shown in Table 2 reveals isometric growth in male L. coubie and in both sexes of L. senegalensis (b values = 3), while there was a negative allometric growth pattern observed for female L. coubie (b=2.63). Mean condition factor for all the fishes were greater than one, with the highest value recorded in male L. senegalensis and the least observed in female L. senegalensis. Sex ratio observed in this study were same as expected ratio 1:1 (Table 3).
| Species |
Sex |
A |
b |
r |
K |
K Mean ± S.E |
| L. coubie |
Male |
1.89 |
3.00 |
0.92 |
2.25 |
1.38 ± 0.42a |
| L. coubie |
Female |
1.41 |
2.63 |
0.91 |
2.16 |
1.23 ± 0.37d |
| L. senegalensis |
Male |
2.01 |
3.07 |
0.95 |
2.45 |
1.28 ± 0.35c |
| L. senegalensis |
Female |
1.94 |
3.04 |
0.95 |
2.12 |
1.33 ± 0.35b |
Table 2: Length-weight relationship and condition factor of Labeo coubie and Labeo senegalensis from lower River Benue.
| Species |
No. Males |
No. Females |
Sex ratio |
| L. coubie |
46 |
54 |
1:1.2 |
| L. senegalensis |
49 |
51 |
1:1 |
Table 3: Sex ratio of Labeo coubie and Labeo senegalensis.
Analysis of stomach content in L. coubie and L. senegalensis (Tables 4 and 5) indicated that most of the samples had above 50stomach fullness as at the time of capture and analysis. Food item isolated in this study basically revealed an omnivorous feeding habit comprising of detritus, algae, insect parts, plant material, mud/sand and some unidentified food items. Mean of percentages between point and frequency of occurrence method used in this study revealed that Algae is most dominant of the feed of male (39%) and female (34) L. coubie, while Detritus dominate the diet of L. senegalensis (42 and 32% for male and female). Aside the unidentified food items in the study, result revealed plant material contribute the least to the nutrition of the fishes (12, 10, 13, and 10% respectively for male and female L. coubie and L. senegalensis).
| Stomach fullness% |
Percentage of stomach sampled sample |
| |
L. coubie |
L. senegalensis |
L. coubie |
L. senegalensis |
| 0 |
13 |
8 |
13 |
8 |
| 25 |
9 |
18 |
9 |
18 |
| 50 |
22 |
19 |
22 |
19 |
| 75 |
30 |
18 |
30 |
18 |
| 100 |
26 |
37 |
26 |
37 |
| Total |
100 |
100 |
100 |
100 |
Table 4: Stomach fullness of Labeo coubie and Labeo senegalensis.
| Food items |
L. coubie |
L. senegalensis |
| |
Male |
Female |
Male |
Female |
| Detritus |
34 ± 0.02b |
28 ± 0.01b |
42 ± 0.01a |
32 ± 0.20a |
| Algae |
39 ± 0.42a |
34 ± 0.04a |
36 ± 0.23b |
30 ± 0.32b |
| Insect part |
20 ± 0.12c |
19 ± 0.11c |
12 ± 0.12e |
10 ± 0.12d |
| Plant material |
12 ± 0.01d |
10 ± 0.01d |
13 ± 0.01e |
10 ± 0.10d |
| unidentified food |
8 ± 0.22e |
6 ± 0.02e |
15 ± 0.02d |
11 ± 0.02d |
| Mud and Sand |
23 ± 0.02c |
20 ± 0.22c |
20 ± 0.10c |
18 ± 0.12c |
Mean in the same column with different superscript differ significantly (P>0.05)
Table 5: Summary of the food items of Labeo coubie and Labeo senegalensi.
Discussion
This study recorded significant differences in length and weight measured for the different sexes of the two species of Labeo. Allendorf et al. (1987), Wimberger (1992) had previously reported that fish demonstrate the greatest variation in morphological traits both within and between populations of the same species than any other vertebrates. Although variation in fishes have been previously linked to geographical and habitat variation (Beacham 1985, Beacham & Murray 1985, Beacham & Withler 1985, Beacham et al. 1988, Lund et al. 1989 and Kinnisonet al. 1998); the variation observed in this study is better explained by differences in genetic component among groups in the environment (Solomon et al., 2015, Umaru et al., 2015, Olufeagba et al., 2015). However, the differences observed between the sexes of the same species in this study may be explained by biological adaptations of the sexes to the prevailing environmental factor. Knowledge of length-weight relationship has numerous practical applications in fisheries biology (Benedito-Cecilio et al., 1997). The lengthweight relationship of fish reported in this study reveals isometric growth for majority of the sampled fish groups, meaning that the weight and length of the fish increases at approximately the same rate, hence the negative allometric growth recorded for female L. coubie implies that the fish becomes more slender as it increase in weight (Riedel et al., 2007). It has been reported by some fish biologists that ‘b’ values usually range between 2.0 and 4.0 for many species (LeCren 1951); this is in line with the findings of this work. Shahririar Nazrul et al., (2011) had previously reported the value of “b” in GIFT and GIFU to be 2.69 and 2.72 respectively, while Narejo et al. (1999) and Al-Baz and Grove (1995) reported value of 2.68 and 3.16 for male and females Tenualosa ilisha (Hamilton, 1822) respectively. The most logical explanation for differences recorded for ‘b’ value in the previous studies cited is largely due to differences in species, strain, stock, sex, and environmental factors. In fisheries science, the condition factor is a term used to compare the “condition of fatness or well-being of the fish” (Shinkafi et al., 2013). It is based on the hypothesis that the heavier the fish of a particular length, the better their physiological condition (Bagenal, 1978). Condition factor is also a useful index for monitoring of feeding intensity, age and growth rates in fish (Oniye et al., 2006), with condition factor of above 1.0 recorded in all the samples, African carp in this river can be said to be in absolutely good condition at the time of sampling.
The stomach content analysis in this study revealed that many of the stomach had above 50% fullness, the availability of food item in relative high amount in the sampled fish can be an indication of efficiency of sampling method used in this study. However, with the omnivorous food items discovered in the fishes it may also be linked to the good feeding habit employed by the fishes, hence enabling them to utilize available food item within the environment. This is similar to the findings of Olele (2011) on feeding habit of Hyperopisus bebe occidentalis Gunther, 1866 caught in Warri River. The 98% gut fullness recoded in Olele’s (2011) study was attributed to the efficiency of the method of feed analysis, which was presumed to have stop food digestion during sampling through the injection of formalin into the gut region of the fish. Furthermore, Haroon, (1998) and Nwani (2004) opined that the greater number of guts with food in their study was as a result of both the feeding strategy adopted by the fishes and the abundance of food during the sampling. The results of this study reveals omnivorous feeding habit of African carp fish, this findings is similar to the report of Olatunde (1989), Adeyemi et al., (2009) and Adeyemi (2010) on the food and feeding habits of Synodontis schall (Bloch and Schneider, 1810) and Synodontis resupinatus Boulenger, 1904 from Zaria area and Idah area of River Niger respectively. The high dominance of detritus and algae in the diet of most sample fish may be an indication of their abundance in the environment and not necessarily the preference of the fish for such food since omnivores have a wild spectrum of food to select from. Adeyemi and Akombo (2012) had earlier stated that plant material extremely dominated the food item (80%) of L. senegalensis in Idah area of lower river Niger. Hence, the spectrum and abundance of food item in the environment under study may determine food preferences of fish in the environment regardless of their feeding habit. In earlier studies by Ayotunde et al. (2007), dietary food of L. cobie were reported to includes whole worm, worm part, nematode, mud, plant part, unidentified items, Rotifera (Kerattela sp., Polyarthera sp. and Philodina sp.), Crustacean (Copepod sp., Decapods sp. and Daphnia sp.) and detritus, many of which were not identified in this study. The ability of both species of fish investigated in this study to feed on a broad spectrum of feed as justified in this study and those of cited literature make them possible aquaculture candidate for domestication. Hence, it is recommended that feeding trial be conducted with commercial and on farm feed stuff using wild caught fingerlings. Some other aspect of the biology of the fish (e.g., reproductive biology) needs to be further investigated so as to understand better the husbandry requirement of the fish for successful culture in captivity.
9183
References
- Adeyemi, S.O., Akombo,PM.(2012) Diet and dietary habits of Labeosenegalensis in a tropical freshwater ecosystem, Animal Research International,9, 1502-1505
- nAdeyemi, S.O. (2010)Food and feeding habits of Synodontisresupinatus (boulenger, 1904) at Idah Area of River Niger,Kogi State,Nigeria.Animal Research International, 7,1281-1286
- nAdeyemi, S.O., Bankole, N.O., Adikwu, I.A., Akombo P.M. (2009) Age, Growth and Mortality of some commercially important Fish Species in Gbadikere Lake, Kogi state, Nigeria.International Journal of Lakes and Rivers,2, 63–69
- nAdeyemo, O.K. (2004)Consequences of pollution and degradation of Nigerian Aquatic Environment on Fisheries Resources.The Environmentalist,23, 297-306
- nAl-Baz, A.F., Grove, D.J. (1995) Population biology of Sbour, Tenualosailisha(Hamilton-Buchanan) in Kuwait.Asian Fisheries Science, 8, 239-259
- nAllendorf F.W., Ryman, N., Utter, F. (1987) Genetics and fishery management: past, present and future in population genetics and fisheries management. Seattle, WA and London: Univ. of Washington Press, pp. 1-20
- nAyotunde, E.O., Ochang, S.N., Okey, I.B. (2007) Parasitological examinations and food composition in the gut of feral African carp, Labeocoubie in the Cross River, Southeastern, Nigeria.African Journal of Biotechnology, 6 , 625–630
- nBagenal, T.B. (1978) Aspects of Fish Fecundity. In: Gerking, S.D. (Ed.), Ecology of Freshwater Fish Production, Blackwell Scientific Publications, Oxford, pp: 75-101
- nBeacham, T.D., Murray, C.B. (1985) Variation in length and body depth of pink salmon (Oncorhynchusgorbuscha) and chum salmon (O. keta) in southern British Columbia. Canadian Journal of Fisheries and Aquatic Sciences,42,312-319
- nBeacham, T.D., Withler, R.E. (1985) Heterozygosity and morphological variability of pink salmon (Oncorhynchusgorbuscha) from southern British Columbia and Puget Sound.Canadian Journal of Genetics and Cytology, 27, 571-579
- nBeacham, T.D., Withler, R.E., Murray C.B., Barner, L.W. (1988) Variation in body size, morphology, egg size, and biochemical genetics of pink salmon in British Columbia. Trans. Amer FishSoc,117,109-126
- nBeacham, T.D. (1985), Meristic and morphometric variation in pink salmon (Oncorhynchusgorbuscha) in southern British Columbia and Puget Sound.Canadian Journal of Zoology,63,366-372
- nBenedito–Cecilio, E., Agostinho A.A., Camelos-Machado-Velho R.C. (1997) Length-weight relationship of fishes caught in the Itaipu Reservoir, Parana, Brazil. NAGA, the ICLARM Quarterly, 1997, 57–61
- nCortes, E. (1997) A critical review of methods for studying fish feeding based on analysis of stomach elasmobranch Fishes, Canadian Journal of Fisheries and Aquatic Sciences, 54, 726-738
- nGulland, J.A. (1983) Fish stock assessment: a manual of basic methods. FAO / Wiley series on Food and Agriculture. Rome
- nHaroon, A.S. (1998) Diet and Feeding ecology of two species of Barbodesgonionotusand Oreochromis species in rice field in Bangladeh.Naga, the ICLARM Quarterly, 13–18
- nHynes, H.B.N. (1950) Food of fresh water stickle backs with a review of methods used in studies of food fish. Journal of Animal Ecology, 19, 36– 58
- nIdodo-Umeh, G. (2005) The feeding ecology of Mochokid species in River Ase, Niger Delta, Nigeria. Trop. Freshwat. Boil,14, 71-93.
- nKing, R.P. (1996) Length-weight relationship of Nigerian Coastal water fishes. NAGA, ICLARM Quarterly, 19,53-68
- nKinnison,M., Unwin,M., Boustead,N., Quinn, T., (1998) Population-specific variation in body dimensions of adult Chinook salmon (Oncorhynchustshawytscha)from New Zealand and their source population, 90 years after introduction. Canadian Journal of Fisheries and Aquatic Science,55, 554-563
- nKulbicki, M., Moutham, G., Thollot, P., Wanteiz, I. (1993) Length-Weight relationship of fish from the lagoon of mew Caledonia.Naga iclarm quarterly, 16, 26 – 30
- nLeCren, E. D., (1951)The length weight relationship and seasonal cycle in gonad weight and condition in the perch (Percafluviatilis). Journal of Animal Ecology, 20, 201-219
- nLund, R.A., Hansen L.P., Jarvi, T. (1989) Identification of reared and wild salmon by external morphology, size of fins and scale characteristics. NINA Forskningsrapp,1, 1-54
- nMendes, B., Fonseca, P., Campos, A. (2004) Weight-length relationships for 46 fish species of the Portuguese west coast. J of ApplIchth, 20, 355-367
- nNarejo, N.T., Ali, S.S., Jafri, S.I.H., Hussain, S.M. (1999) A study on the age and growth of Palla, Tenualosailishafrom the River Indus. Pakistan Journal of Zoology, 31,25-29
- nNwani, C. D. (2004) Aspects of the biology of mormyrids in Anambra River, Nigeria.Ph.D Thesis University of Nigeria Nsukka 194 pp
- nOkafor, A.I., Egonmwan, R.I., Chukwu, L.O. (2012) Behavioural Ecology of the African Lungfish, Protopterusannectens (Owen, 1839) of Anambra River, Nigeria. International Journal of Environmental Biology,2, 208-214
- nOlatunde, A.A. (1989) Some Aspects of Biology of Synodontisschall (Bloch andSchneider, 1801) in Zaria, Nigeria. Journal of Aquatic Sciences, 4, 49–54
- nOlele, N.F. (2011) Diet Composition, Length/Weight Relationship and Condition Factor of Hyperopisus bebe occidentalis (Lacepede 1803) Caught in Warri River. Journal of Basic Applied Science Research, 1, 9,998-1005
- nOlufeagba, S.O., Aladele, S.E., Okomoda, V.T., Sifau, M.O., Ajayi, D.A., et al. (2015) Morphological Variation of Cichlids from Kainji lake, Nigeria. Journal of Fisheries Science.com, 9, 70-80
- nOniye, S.J., Adebote, D.A., Usman, S.K., Makpo, J.K. (2006) Some aspects of the biology of Protopterus annectens (Owen) in Jachi Dam near Katsina, Katsina state, Nigeria. Journal of Fisheries and Aquatic Research, 1, 136-141
- nPauly, D, (1983) Some simple methods for the assessment of tropical fish stocks. FAO Fisheries Technical paper, (234), FAO Rome, Italy.
- nPius, M.O., Benedict, O.O. (2002) Food and feeding inter-relationship.A preliminary indicator to the formulation of the feed of some Tilapine fishes.Tropical journal of animal science, 5, 35-41
- nRahman, A.K. (1989) Freshwater Fishes of Bangladesh. Zoological society of Bangladeh, 4, 177-180
- nRiedel, R., Caskey, L.M., Hurlbert, S.H. (2007) Length weight relations and growth rates of dominant fishes of the Salton Sea: implications for predation by fish-eating birds. Lake and Reservoir Management,23, 528-535
- nShahriar Nazrul, K.M., Mamun, A.A., Sarker, B.S., Tonny, U.S, (2011) Morphological variability of the 11th generation strain of nile tilapia, (Oreochromis niloticus) and traditional genetically improved farmed tilapia. Journal of Bangladesh Agricultural University,9,345-349
- nShinkafi, B.A., Ukoha L.N., Tukur N.A. (2013) Some Aspects of Growth and Reproduction in the Nile Perch (Lates niloticus, Linne 1762) From River Rima, North-western Nigeria, Nigerian Journal of Fisheries and Aquaculture,1, 31-41
- nSolomon, S,G., Okomoda, V.T., Ogbenyikwu, A.I. (2015) Intraspecific morphological variation between cultured and wild Clarias gariepinus (Burchell) (Clariidae, Siluriformes). Archives of Polish Fisheries,23, Pp 53-61
- nSolomon S.G., Okomoda V.T., Aladi, S.L. (2012)Fish fauna in lower River Niger at Idah in Kogi State. Journal of Agricultural and Veterinary Sciences,4, 34-37
- nSumbuloglu, K, Sumbuloglu V. (2000)Biyoistatistik.HatipogluYayinlari, 53, 269
- nUmaru J A, Annune P A, Cheikyula J O,Okomoda V T, (2015) Some Biometric Parameters of Four Selected Fish Species in Doma Dam, Nasarawa State, Nigeria,International Journal of Aquaculture,5, 1-7
- nWimberger, P.H. (1992) Plasticity of fish body shape, the effects of diet, development, family and age in two species of Geophagus (Pisces: Cichlidae). Biological Journal of Linnean Society,45, 197-218
- nWootton, R.J. (1992) Fish ecology.Blackie and Son Limited. Chapman and Hall, New York. 200 Pp.