Keywords
Shi drum, Umbrina cirrosa, GnRHa, spawning behaviour, hatching rate
Introduction
The shi drum (Umbrina cirrosa, Linnaeus 1758), a member of the Sciaenidae family, is a valuable species for aquaculture in the Mediterranean. The cultivation methods and techniques have been under development for the last few years. There has been an interest in spawning and cultivation of this species as a potential candidate for commercial aquaculture, because of its high growth rate, high market price and good adaptability to culture conditions (Melotti et al., 1995; Barbaro et al., 1996; Libertini et al., 1998; Mylonas et al., 2000; Koumoundouros et al., 2005). Reproductive biology of the shi drum has attained much attention and some research was carried out by Mylonas et al. (2000; 20004), Melotti et al. (1995), and Barbaro et al. (1996) in captive conditions. Mylonas et al. (2000) have described some behavioral features for application in production. These include inducing with a hormonal treatment for spawning in captivity. Methods for effective hormone application in several genera of fish that require long-term stimulation of LH release in final oocyte maturation, ovulation and spawning have been well documented (Mylonas et al., 1998).
Determination of spawning behaviour of new fish species is crucial for broodstock management and obtaining viable gametes. The final stage of a reproductive period resulting in spawning can be controlled by either placing the fish in a favourable environment or by altering the fish’s internal regulating factors with injected hormones (Mugnier et al., 2000; Forniés et al., 2001; Mylonas and Zohar, 2001; Coward et al., 2002). The internal mechanisms that regulate spawning are similar for most fishes (Patino, 1997; Zohar and Mylonas, 2001). The external environmental factors control reproduction, however, vary considerably among species (Donaldson, 1996; Devlin and Nagahama, 2002). There are numerous studies about the internal regulatory mechanism of fish reproduction and the specific environmental requirements for spawning. But few studies have examined the behavioural pattern of breeders in spawning period (Olsén et al., 1998; Kobayashi and Nakanishi, 1999; Volkoff and Peter, 1999; Cheek et al., 2000). The observations of spawning behaviour in aquaculture have been utilized to solve some bottlenecks which restrict the amount of production in captive fish (Melotti et al., 1995; Olsén et al., 1998; Okumura et al., 2002; Mizuta and Kubokawa, 2004).
In the present study, we evaluated the effect of hormone injection and delivery systems impregnated with gonadotropin releasing hormone (LH-RHa) on spawning induction and hatching in the shi drum Umbrina cirrosa. Furthermore, spawning behaviour was observed and recorded on video camera prior and during spawning in captivity.
Materials and Methods
The study was carried out during the spawning season of the shi drum broodstocks kept in indoor tanks under culture conditions in a commercial hatchery July 2006 (Water temperature: 19-24 ºC; Salinity: 39-40 p.p.t.).
Experimental animals
Shi drum juveniles were obtained from the lagoon area of Söke, called Carina (37° 35' N latitude, 27° 13' E longitude), by beach seine in August 2003. A total of five operations were performed. After each fishing operation, living fish were transferred inshore in two 40-L plastic bags and then, they were placed in two transporting tanks (1 m3 each) on truck. These tanks were supplied with liquid O2, and used to transport the juveniles to the Akva-Tek fisheries company in Zeytinda?, Bergama, Izmir, on the central west cost of Turkey, where the experimental trials took place. During the following three years all the fish were maintained in outdoor 20 m3 circular polyester tanks. In the first year the fish were daily fed ad libitum with fresh seafood (small fish, chopped squid and crustacean). In the second year they were fed twice a day, six days a week with prepared raw diets (containing fish flour, fish oil, fresh squid, fresh fish, crustacean and mix vitamins) and maintained under simulated natural photoperiod (38° 55' N latitude, 27° 03' E longitude) and temperature until the time of the experiment. During this period the water temperature ranged between 11 ºC and 28 ºC.
Experiments started in July 2006 when the fish were 3+ years old and when the temperature was 19 ºC. Ripe females were selected from the holding tank (n=108) after lightly anesthetization with 0.05 mlL-1 2-phenoxyethanol. The selected brood fish were then placed in a separate bucket where they were deeply anesthetized with 0.3 mlL-1 2-phenoxyethanol (Mylonas et al., 2004).
Flowing males were selected by gentle abdominal pressure. Only the females for which ovarian biopsies could be performed were chosen. The sampled oocytes were placed immediately under the light microscope where the diameters (μm) of 30-40 oocytes per fish were measured. The weight (g) and total length (cm) of 49 selected fish were measured and recorded. The brooders were then divided into three groups as follows: Control (6 females and 7 males), group I (12 females and 10 males), and group II (6 females and 8 males). Each group was placed in three 4-m diameter x 1.0-m deep cylinder polyester tanks (approximately 12 m3), equipped with overflow egg collectors.
Hormone administration
One day after the selection, hormone administration was performed on the females only. The females in the control were injected intramuscularly with saline solution. The females of Group I were injected with 10 μgkg-1 body weight with GnRHa (LH-RHa, Argent Labs, U.S.A.) two days after selection and again 5 days later. Females of Group II were implanted with a sustainable- released cholesterol pellet loaded with GnRHa. Fish which weighed 2 kg were implanted with a single pellet (approximately 50 μgkg-1 bw); those which weighed less than 2 kg with less than one pellets. The cylindrical pellets (2 mm diameter x 6 mm long, 30 mg) were prepared within a pellet press (Mylonas and Zohar, 2001) and contained 85 % cholesterol (Wako, Japan), 15 % cocoa butter and 100 μg of GnRHa. Both the GnRHa injections and the GnRHa pellets were administered intramuscularly. After treatment, fish were placed separately from the rest of the broodstock and were allowed to spawn spontaneously in 12 m3 tanks fitted with overflow egg collectors. Fish were not disturbed until the termination of the experiment.
Spawning Behaviour
The observation of spawning behaviors was carried out daily after the administration of hormones. A video camera (Panasonic, NV-DS50A, Matsushita Ind., Osaka, JAPAN) used to record the spawning behavior was mounted on a tripod in front of the tank. It was located 30 cm above the top of the tanks’ sides. The field of the view included almost the whole tank and it focused on the center of the background of the tank.
Every morning (1 h. after the lights switched on), first observation was performed in control group and video recording was taken for 5 min. Then the other group’s recordings were done with the same video camera for 5 min sequentially. During the light hours, fish were observed from the corner of the tank without being disturbed and their activities were noted. Spawning behaviour was recorded easily whenever the unexpected activity in the observation tanks was noticed and these activities were recorded immediately. After each recording of spawning, egg collectors were checked within an hour and correlated with the presence or absence of spawned eggs in the egg collector. A total of three hundred and sixty minutes of video recordings were carried out during the experiment for all the trial tanks. Each tape was viewed in the laboratory and all different behavioral patterns were noted. The general swimming patterns in all groups were evaluated. The feeding behaviour and aggressive movements of the broodstock were also caught on the TV monitor. In addition, the signals of the breeding time were noted for potential improvements of the broodstock management.
Egg quality
The eggs were collected from each tank four times a day (at 08:30, 13:00, 19:30, 23:30) and weighed separately. Furthermore, when the spawning behaviors indicated a possible spawning then the egg collectors were checked within the following hour and results were noted. The eggs were classified as viable if they showed a spherical shape, translucent appearance and lack of perivitelline space. Unfertilized dead eggs were separated from the bottom of the transparent container with siphon. The incubation of fertilized eggs from each group was carried out separately in a hatching system containing four tanks (4 m3) with closed recirculation system with 20% per hour water renewal. The incubation was performed in 50 l cylindroconical incubators with aeration in a 4 m3 tank, and hatching rates were determined. Diameters of 50 randomly chosen viable eggs were measured by using a microscope with a calibrated eyepiece. The main developmental stages were photographed under the microscope and the timing was recorded. For the calculation of volume of yolk sac and lipid droplet the formula of Rosa and Dinis (1985) and Klaoudatos et al. (1990), were used, respectively.
Statistical Analysis
The significance tests of the normal distribution of the total lengths and weights of females and males were done by using one-sample Kol mogorov Smirnov’s, and homogeneity tests were done by using Levene’s statistical analysis. The significant tests of the differences between the mean total lengths and weights of these fish were compared with independent sample t-test. The average diameter of the obtained eggs was compared with independent sample t-test between the two hormone induced groups. One-way analyses of variance (ANOVA) was applied to determine the differences in the eggs diameters in each group according the days and followed by Tukey HSD test was used to find daily differences in the group. Pearson’s χ2 test was applied to find whether there was a statistical difference between the hormone application groups about the fertilization rate of total obtained eggs during the experimental period. All statistical analyses were performed by using SPSS software (version 9.05) and Microsoft Excel software (version 2002) and significance was accepted at p = 0.05.
Results and Discussion
Animals
Table 1 shows biometric values of the threeyear- old experimental animals used in this experiment. The weight and total lengths between the female (a=0.009, b=39.31, R2=0.57) and male (a=0.009, b=38.62, R2=0.75) were not significantly different (p>0.05, independent sample ttest). Only 49 fish were selected among 108 specimens for the experiment. Only the three year old fish were chosen. The others did not show sexual maturity as checked by either a biopsy or a gentle pressure on the abdomen.
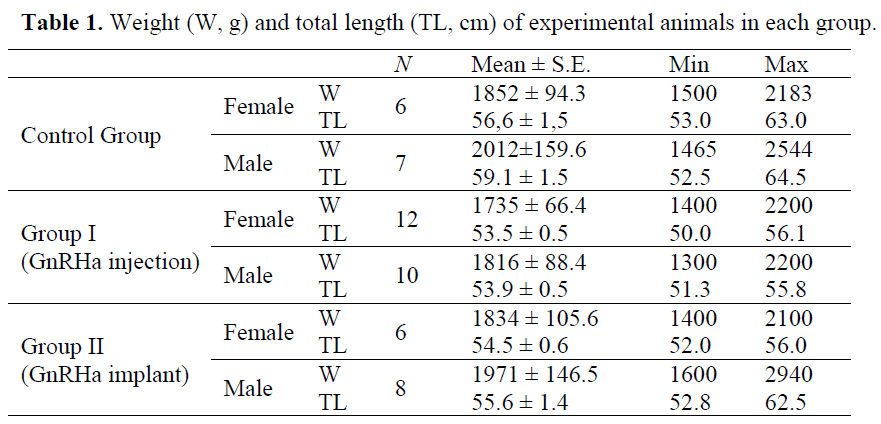
Table 1. Weight (W, g) and total length (TL, cm) of experimental animals in each group.
Hormone application
Spontaneous spawning was not observed in the control group during the entire experimental period, but the hormonal treatment group did spawn successfully. While hormone implant group spawned after 4 days and hormone injection group spawned after 3 days. The first released eggs were obtained in 70-72 h later at 14.00 p.m. in group I and lasted for 7 days and then stopped. In group II, eggs were released in 89-90 h at 09.00 a.m. and lasted for 8 days (Table 2). Results indicate that the group with a hormone injection induced spawning one day faster than the hormone implanted group.
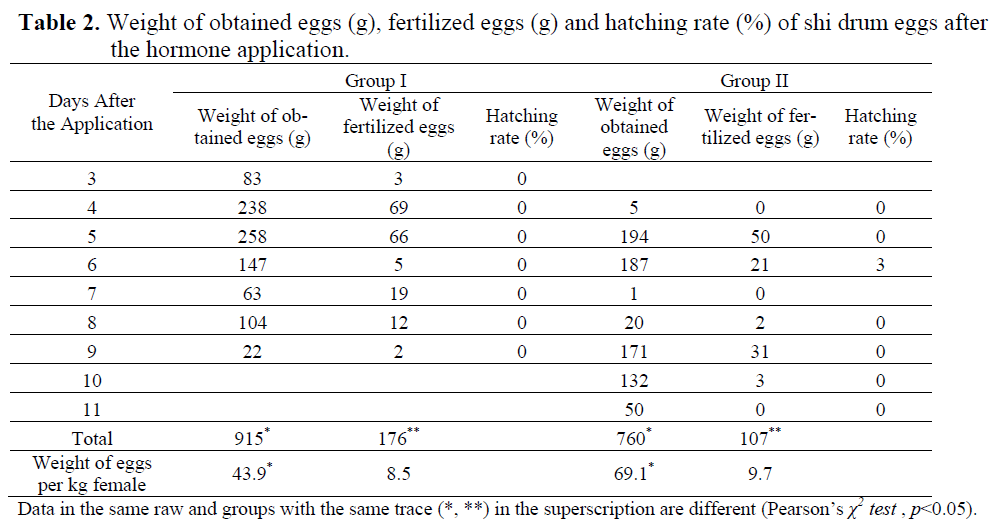
Table 2. Weight of obtained eggs (g), fertilized eggs (g) and hatching rate (%) of shi drum eggs after the hormone application.
Spawning behaviour
Males and females of shi drum school in the culture tank during the daylight. Generally, they swim up near the edge of the tank in parallel to this they swim up from the bottom to the surface of the water and their dorsal fins show easily above of the water. Then they return to the bottom of the tank and they always swim close to the edge of the cylindrical tank. In normal life period, it isn’t possible to see the difference between males and females because both are the same size. However, after the hormone administration, sex differentiation is easily predictable. The abdominal side of the treated females starts swelling. Except for the feeding time, no competitive behaviors are observed and the fish compete only during feeding.
After hormone administration, generally spawning was observed in the following 7-8 days of the 30 days of video recordings. Spawning behaviors were succesfully recorded in daylight period. In both of the hormone treated groups spawning occurred during the night while there was no video recording. About 65% of the releaseed eggs were present in the egg collectors in the mornings.
Compared with the control, hormone induced groups changed remarkably in their behaviors almost immediately. The changes in reproductive behavior were seen within 20 and 24 hours in the injected and implanted groups, respectively. The spawning pattern of shi drum was considered to be synchronous as males and females of the both hormone induced groups initiated spawning around 70-90 h after the application. Males were easily distinguished from females which had visible swelling associated with ovulation and hydration. Several males were seen swimming vigorously while pursuing a female, swimming parallel to her, and following her closely. During this time, the abdominal areas of the females were visibly swollen and females were chased by one male but sometimes two or three males depending on the time of release.
Aggregation behaviour was seen among the males. Two or three males swam right behind a female competing among themselves to be closer to the swollen female. At this time, they push and shove one another using their heads for winning a better spot in the competition. This competition lasted approximately half an hour. At the end, generally one female and one male swam with the male fish always following the female. Before the actual spawn, the brooders swam towards the bottom of the tank, closely synchronizing their body movements. At times, the pair stopped in the middle of the tank for a short time and female was seen inclining her head down while releasing the eggs. .At the same time, the males laid on there side close to the female’s lifted abdomen while releasing their sperm. Although male aggregation behavior wasn't observed in the control group, both of the induced groups showed clear signals of spawning activities.
Fish in the control group were fed continuously except for two days of experimental manipulation and the following day. Grouping behavior was not observed in this group during the whole experimental period. Fish in the injected group were not fed after the experimental manipulation and following ten days. Fish in implanted group also were not fed after the experimental manipulation and following 12 days. Similar behavioral features were observed in the implant group like the ones in the injected group.
A clear indication of spawning was observed on the water surface of egg collector 6-10 h. prior to the appearance of fertilized eggs in the collectors. Yellowish and oily foam was accumulated on the surface water of the egg collectors. Although it is widely observed in hatcheries, the timing of the foaming of the egg collector has not been reported before. Except for control group, results indicate that fish induced with GnRHa can give some signals before the spawning day. We can determine the spawning time if we observe the brood fish more carefully.
Egg quality
At the first sampling at the beginning of July, the mean diameter (S.E) of the oocytes was 444.08 ± 6.02 μm in control group (n=114), 440.28 ± 5.02 μm in group I (n=138) and 454.98 ± 4.97 μm in group II (n=121). There were no significant differences among the size of oocytes (p>0.05, ANOVA). GnRHa stimulated spawning in both hormone application groups. First ovulated eggs (mean ± S.E.; 759.3±1.2 μm) were obtained in Group I on 3rd day after the hormone application and its fertilization rate was only 3.49 %. In Group II, first eggs (mean ± S.E.; 725.0±3.6 μm) were obtained on 4th day after the hormone application and no fertilization was observed (Table 2 and 3). The mean diameter of the total eggs was 767.5±1.0 μm in Group I and 753.5±1.3 μm in Group II (Table 3). Significant differences were found among the total egg diameters (p<0.05, Independent Samples t-test). There were significant differences in the amounts of total eggs and fertilized eggs between the hormone induced groups (p<0.05, Pearson’s χ2 test). The total released eggs per kg female of Group II were heavier than those of Group I and hatching was only carried out with the eggs obtained on 6th day in the implanted group.
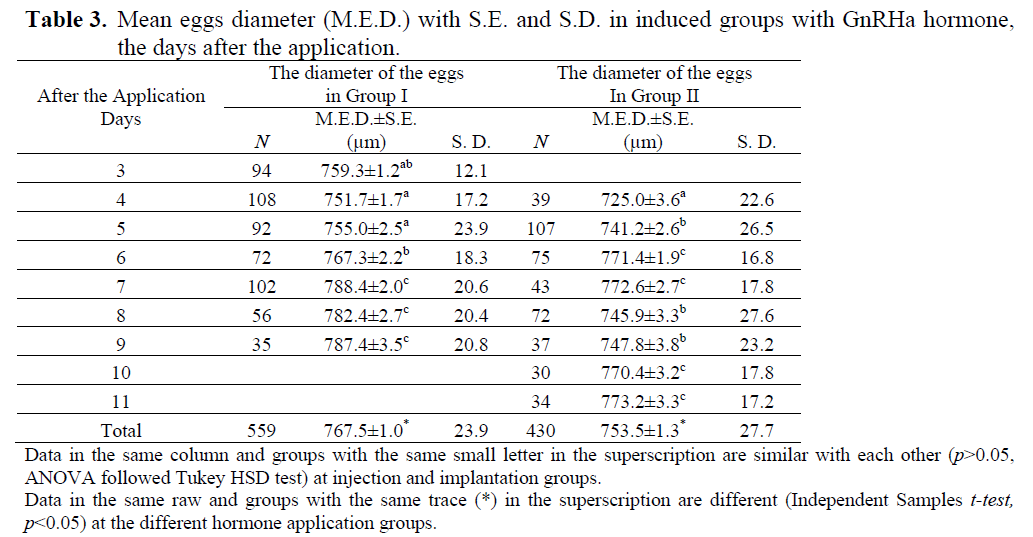
Table 3. Mean eggs diameter (M.E.D.) with S.E. and S.D. in induced groups with GnRHa hormone, the days after the application.
Shi drum eggs are spherical, buoyant, transparent, and have a clean chorine. In the centre of eggs there is an oil droplet. The amount of spawned eggs and fertilized eggs per kg female were found to be 43.9 g and 8.5 g in injected group and 69.1 g and 9.7 g in implanted group, respectively. Although there were no significant differences in fecundities, fertilization rates were markedly different (p<0.05, p>0.05, Pearson’s χ2 test, respectively). After 1.5-2.0 hours of fertilization, two-cell stage development of the fertilized eggs was observed. Gastrula stage, first heart beat of embryo, and hatching of eggs was observed at 15-17 h, at 32-33 h, and 47-48 h after fertilization at 19.0±0.5°C, respectively. At the beginning of the larval phase, shi drum had a large yolk sac and a single lipid globule. The total length (mean ± S. D.) of first larvae was measured with 1.855 ± 0.066 mm (n=10), the volume of yolk sack and lipid droplet were estimated 0.061 ± 0.021 and 0.006 ± 0.001 mm3, respectively.
One of the requirements in improving a new species for aquaculture is obtaining reliable amounts of viable eggs (Dumas et al., 2004) and it is often necessary to use exogenous hormone treatment to stimulate ovulation (Morehead et al., 1998; Zohar and Mylonas, 2001). Induction of ovulation by gonadotropin-releasing hormone analogue (GnRH-a) is a general procedure for controlling reproduction of commercial aquaculture (Crim et al., 1987; Mugnier et al., 2000; Zohar and Mylonas, 2001). This phenomenon was supported by Mylonas et al. (2004) findings in shi drum as well. Their study showed that they hadn’t obtained ovulated eggs from the control groups weighing 2.1 kg in July of 2001 and spawning season of 2002. In the present study, similar results of first spawning season of threeyears- old shi drum were found. The average diameters of biopsied oocytes were 446.21 ± 3.08 μm in all groups (n=373). The weights of females in control group ranged between 1.4 and 2.1 kg. In our study neither ovulation nor fertilization was recorded for the control group.
In cultured marine species, reproductive dysfunction is commonly encountered. This could be caused collectively or individually by no final oocytes maturation (FOM), ovulation and/or spawning (Mylonas et al., 2000; Mylonas and Zohar, 2001). Many researchers studied different spawning methods to supply healthy fertilized eggs before they started to produce the larval fish (Mugnier et al., 2000; Barbaro et al., 2002; Mylonas et al., 2004). There have been several methods used for induction of spawning in shi drum. Two induction methods containing injection of GnRHa and GnRHa implant using cholesterol as binder were used. Hatching rate was recorded only 3 % in the group of GnRHa implant, prepared with cholesterol and cocoa butter. Contrary to Barbaro et al. (2002) and Mylonas et al. (2004), hatching of the eggs didn’t accomplish in injection group. But it was thought that the weights of their experimental fish were bigger than broodstock in this study and it is possible to obtain better results using injection of hormone. A plausible explanation could be related to the stress of capture of our spawners. Cholesterol pellets were found to be more useful than the direct injection of hormone in the first phases of sexual maturity in shi drum.
It has been well documented that many fish species used conspecific odors of pheromone for gender recognition, synchronization of spawning, and reproductive behavioral responses (Stacey, 2003; Sorensen et al., 2005; Huertas et al., 2006; Dou et al., 2007). In this study, the females were stimulated with hormone and spawning activity started 20-24 hours. Meanwhile males were clearly ready for insemination of the ovulated eggs without any hormonal treatment. This could suggest that shi drum odors released by mature females could serve as short-range attractants that affects the spawning activity of the males. Same observations were found in goldfish Carassius auratus and reported that reproductive females released a prostaglandin F2α pheromone, which acted in the brain to stimulate female sex behavior and male courtship and spawning activities (Kobayashi et al., 2002).
Although a few studies reported information on spawning behaviors’ of other finfish in captivity, there wouldn’t find any information related to the characteristics of behaviors of shi drum in the spawning time. The swimming patterns while spawning differs significantly among species (Baynes et al., 1994; Olsén et al., 1998; Okumura et al., 2002; Mizuta and Kubokawa, 2004; Esteve, 2005). The swimming patterns during spawning were observed to be similar in both hormone treated groups. Spawning behavior of shi drum was observed after 20 h in injection group and 24 h in implant group and swimming in pairs was lasted until the end of the egg release. On the other hand, no apparent swimming pattern was seen in the control group. Although the act of egg release could not be seen on the recordings as it occurred in darkness, the courtship between female and male resulted in spawning and fertilization indicated by the eggs in the collectors within less than an hour.
It was clearly demonstrated that the first spawning was triggered by a hormonal treatment on the three year-old shi drum. The spawning time can be predicted by observing the swimming patterns of the broodstock. In this study, a clear evolutionary pattern was observed during courtship and spawning of shi drum. A successful reproduction of shi drum followed a pathway sequentially passing through four categories namely, stimulation of males, courtship of several competing males, resilience of the strongest male and finally insemination of the released eggs by the strongest male. The video recordings indicated that shi drum females were inseminated by the strongest males in the group following a long chase in the culture tanks. Close courtship of male and female is characteristic of the other fishes where spawning behaviour has been described (Konstantinou and Shen, 1995; Smith et al., 1999; Pankhurst and Fitzgibbon, 2006).
The use of GnRHa was found to be effective in completing FOM and ovulation in shi drum in captivity. It is possible to achieve ovulation by using cholesterol pelleted with GnRHa in early period of spawning age. It can potentially be used in the reproduction and production of new alternative fish species. Observation of spawning behavior can be a useful tool to determine the spawning time in commercial production.
Acknowledgements
We are indebted to Dr. Huseyin Ozbilgin for helpful comments and MSc. Bilhan Filizkan, MSc. Serkan Ilgaz, and Alparslan Kilic for providing logistical assistance. This work was funded by AKVA-TEK Fisheries Company.
1481
References
- Barbaro, A., Francescon, A., Bertotto, D., Bozzato, G., Di Maria, I., Patarnello, P., Furlan, F., Colombo, L., (2002). More effective induction of spawning with long-acting GnRH agonist in th shi drum, Umbrina cirrosa L (Sciaenidae, Teleostei), a valuable candidate for Mediterranean mariculture, Journal of Applied Ichthyology, 18: 192-199.
- Baynes, S.M., Howell, B.R., Beard, T.W., Hallam, J.D., (1994). A description of spawning behaviour of captive dover sole, Solea solea, Netherlands Journal of Sea Research, 32: 271-275.
- Cheek, A.O., Thomas, P., Sullivan, C.V., (2000). Sex steroids relative to alternative mating behaviors in the simultaneous hermaphrodite Serranus subligarius (Perciformes: Serranidae), Hormones and Behavior, 37: 198–211.
- Coward, K., Bromage, N.R., Hibbitt, O., Parrington, J., (2002). Gamete physiology, fertilization and egg activation in teleost fish. Reviews in Fish Biology and Fisheries, 12: 33-58.
- Crim, L.W., Peter, R.E., Van Der Kraak, G., (1987). The use of LHRH analogs in aquaculture. pp. 489-498. In B.H. Vickery and J.J. Nestor (eds.). LHRH and Its Analogs: Contraceptive and Comparative Endocrinology, MTP Press, Boston.
- Devlin, R.H., Nagahama, Y., (2002). Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture, 208: 191-364.
- Donaldson, E. M., (1996). Manipulation of reproduction in farmed fish, Animal Reproduction Science, 42: 381–392.
- Dou, S.Z., Yamada, Y., Okamura, A., Tanaka, S., Shinoda, A., Tsukamoto, K., (2007). Observations on the spawning behaviour of artificially matured Japanese eels Anguilla japonica in captivity. Aquaculture, 266:117- 129.
- Dumas, S., Rosales-Velazquez, M.O., Contreras- Olguýn, M., Hernandez-Ceballos, D., Silverberg, N., (2004). Gonadal maturation in captivity and hormone-induced spawning of the Pacific red snapper Lutjanus peru. Aquaculture, 234: 615-623.
- Esteve, M., (2005). Observations of spawning behaviour in Salmoninae: Salmo, Oncorhynchus and Salvelinus. Reviews in Fish Biology and Fisheries, 15: 1-21.
- Forniés, M.A., Mananos, E., Carrillo, M., Rocha, A., Laureau, S., Mylonas, C.C., Zohar, Y., Zanuy, S., (2001). Spawning induction of individualized European seabass females (Dicentrarchus labrax) using different GnRHa-delivery systems. Aquaculture, 202: 221-234.
- Huertas, M., Scott, A.P., Hubbard, P.C., Canário, A.V.M., Cerdà, J., (2006). Sexually mature European eels (Anguilla anguilla L.) stimulate gonadal development of neighbouring males: possible involvement of chemical communication. General and Comparative Endocrinology, 147: 304–313.
- Klaoudatos, S., Tsevis, N., Conides, A., (1990). Energy sources during the early larval development of the European sea bass, (Dicentrarchus labrax), Aquaculture, 87: 361-372.
- Kobayashi, M., Nakanishi, T., (1999). 11-Ketotestosterone induces male-type sexual behavior and gonadotropin secretion in gynogenetic crucian carp, Carassius auratus langsdorfii. General and Comparative Endocrinology, 115: 178–187.
- Kobayashi, M., Sorensen, P.W., Stacey, N.E., (2002). Hormonal and pheromonal control of spawning behaviour in the goldfish. Fish Physiology Biochemistry, 26: 71–84.
- Konstantinou, H., Shen, D.C., (1995). The social and reproductive behaviour of the eyed flounder Bothus ocellatus, with notes onthe spawning of Bothus lunatus and Bothus ellipticus. Environmental Biology of Fishes, 44: 311-324.
- Koumoundouros, G., Kouttouki, S., Georgakopoulou, E., Papadakis, I., Maingot, E., Kaspiris, P., Kiriakou, Y., Georgiou, G., Divanach, P., Kentouri, M., Mylonas, C.C., (2005). Ontogeny of the shi drum Umbrina cirrosa (Linnaeus 1758), a candidate new species for aquaculture. Aquaculture Research, 36: 1265-1272.
- Melotti, P., Roncarati, A., Gennari, L., Mordenti, O., (1995). Trials of induced reproduction and larval rearing of curb (Umbrina cirrosa L). Oebalia, 21: 37-42.
- Mizuta, T., Kubokawa, K., (2004). Non-synchronous spawning behaviour in laboratory reared amphioxus Branchiostoma belcheri Gray. Journal of Experimental Marine Biology and Ecology, 309: 239-251.
- Morehead, D.T., Pankhurst, N.W., Ritar, A.J., (1998). Effect of treatment with LHRH analogue on oocyte maturation, plasma sex steroid levels and egg production in female striped trumpeter Latris lineata (Latrididae). Aquaculture, 169: 315–331.
- Mylonas, C.C., Woods, L.C., III Thomas, P., Zohar, Y.,1(998). Endocrine profiles of female striped bass (Morone saxatilis) during postvitellogenesis, and induction of final oocyte maturation via controlled-release GnRHadelivery systems. General and Comparative Endocrinology, 110: 276-289.
- Mylonas, C.C., Georgiou, G., Stephanou, D., Atack, T., Afonso, A., Zohar, Y., (2000). Preliminary data on the reproductive biology and hatchery production of the shi drum (Umbrina cirrosa) in Cyprus. In: B. Basurco (eds.). Chaiers Options Méditerranéennes, Mediterranean Marine Aquaculture Finfish Species Diversification. CIHEAM, Zaragoza Spain. 312 pp.
- Mylonas, C.C., Zohar, Y., (2001). Use of GnRHa-delivery systems for the control of reproduction in fish. Reviews in Fish Biology and Fisheries, 10: 463-491.
- Mylonas, C.C., Kyriakou, Y., Sigelaki, I., Georgiou, G., Stephanou, D., Divanach, P., (2004). Reproductive biology of the shi drum (Umbrina cirrosa) in captivity and induction of spawning using GNRHA. The Israeli Journal of Aquaculture-Bamidgeh, 56: 77-94.
- Mugnier, C., Guennoc, M., Lebegue, E., Fostier, A., Breton, B., (2000). Induction and synchronization of spawning in cultivated turbot (Scophthalmus maximus L) broodstock by implantation of a sustained-release GnRH-a pellet. Aquaculture, 181: 241-255.
- Pankhurst, N.M., Fitzgibbon, Q.P., (2006). Characteristics of spawning behaviour in cultured greenback flounder Rhombosolea tapirina. Aquaculture, 253: 279-289.
- Patino, R., (1997). Manipulations of the reproductive system of fishes by means of exogenous chemicals. The Progressive Fish-Culturist, 59: 18-128.
- Rosa, P.H.C., Dinis, M.T., (1985). Diet rhythyms in Dicentrarchus labrax (L, 1758) larvae under controlled conditions swim bladder inflation, feeding and otolith growth. Investigation Pesquera, 49: 3-13.
- Smith, T.I.J., McVey, D.C., Jenkins, W.E., Denson, M.R., Heywood, L.D., Sullivan, C.V., Berlinsky, D.L., (1999). Broodstock management and spawning of southern flounder, Paralichthys lethostigma. Aquaculture, 176: 87– 99.
- Sorensen, P.W., Pinillos, M., Scott, A.P., (2005). Sexually mature male goldfish release large quantities of androstenedione into the water where it functions as a pheromone. General and Comparative Endocrinology, 140: 164– 175.
- Stacey, N., (2003). Hormones, pheromones and reproductive behaviour. Fish Physiology and Biochemistry, 28: 229–235.
- Okumura, S., Okamoto, K., Oomori, R., Nakazono, A., (2002). Spawning behaviour and artificial fertilization in captive reared red spotted grouper. Epinephelus akara, Aquaculture, 206: 165-173.
- Olsén, K.H., Järvi, J.T., Mayer, I., Petersson, E., Kron, F., (1998). Spawning behaviour and sex hormone levels in adult and precocious brown trout (Salmo trutta L) males and the effect of anosmia. Chemoecology, 8: 9–17.
- Volkoff, H., Peter, R. E., (1999). Actions of two forms of gonadotropin releasing hormone and a GnRH antagonist on spawning behavior of the Goldfish Carassius auratus. General and Comparative Endocrinology, 116: 347–355.
- Zohar, Y., Mylonas, C.C., (2001). Endocrine manipulations of spawning in cultured fish: from hormones to genes. Aquaculture, 197: 99–136.









