Keywords
Biofloc, Shrimp, Fishmeal, Fisheries, Alternative, Sustainability
Introduction
In the 10 years, aquaculture production in Egypt showed rapid growth to meet seafood de-mand due to shortage supply of fish and crusta-cean from overexploited fisheries in Egypt. In 2013 more than 75% of seafood supply in Egypt comes from aquaculture and less than 25% comes from capture fisheries. This rapid expansion of aquaculture to meet seafood supply in Egypt re-quires the use of several resources namely; seed for pond stocking and least cost- high quality- feeds. Intensification of aquaculture production cannot depend on fishmeal from natural fisheries which is both overexploited and costly source. For the country to continue in increasing aquacul-ture production, it requires sustainable alternative source to fishmeal in shrimp feeds, otherwise shrimp farmers will be out of business.
In order for aquaculture to be completely suc-cessful, the industry will need to develop tech-nology that will increase economic and environ-mental sustainability (Kuhn et al., 2010). This technology implements cheaper alternative in-gredient to fishmeal and this will effectively re-duce the costs of feed as feed costs can account for more than 50% of operational expense, while reducing their impact on overexploited natural fisheries (Naylor et al., 2009). Thus, it is im-portant to determine if alternative source, biofloc, could be a suitable replacement ingredient in ma-rine shrimp diets. If implemented successfully, this option would offer a sustainable option to fishmeal. Over the period of January 2008 to May 2009, the global fishmeal market varied from a low mean of about $900 to a high mean of $1250 per metric ton. During the same time frame, soybean meal varied approximately from a low mean of $375 to a high mean of $550 (FAO, 2009). Thus, the use of biofloc represents a via-ble and more sustainable feed option due to cost, the manner in which it is generated, and the po-tential that it can decrease the pressure on wild fisheries by reducing at least some of the demand for fishmeal (Avnimelech, 2005).
The bioflocs technology (BFT) is a sustaina-ble technique used in aquaculture to maintain good in situ water quality through the develop-ment and control of dense heterotrophic microbi-al bioflocs by adding carbohydrate to the water (Crab et al., 2010). If organic carbon and nitro-gen are well balanced in a culture system, ammo-nium and nitrogenous waste will be converted into bacterial proteinaceous biomass (Schneider et al., 2005). Hence, developing and controlling dense heterotrophic microbial bioflocs in the wa-ter column by adding carbohydrates improves the water quality in the pond and in addition to this, the bioflocs can subsequently be consumed and used as a source of feed by the cultivated aquatic organisms (Avnimelech, 2005). The aim of this study was to find out sustainable and cost-effective alternative source for costly and over-exploited fishmeal in the feed of marine shrimp.
Materials and Methods
Experimental set-up:
This study was carried out at the Shrimp and Fish International Company (SAFICO), South Sinai, Egypt in the year 2010.The study was car-ried out on (F1) generation produced at SAFICO from May 2010 till October 2010. The descrip-tion of the different treatments (Table 1, Figure 1) and control used is as follow:
A) Control: artificial feed with 45 % CP.
B) Treatment A: artificial feed with 30.10 % CP+ Rice bran.
C) Treatment B: artificial feed with 25.20 % CP + Rice bran.
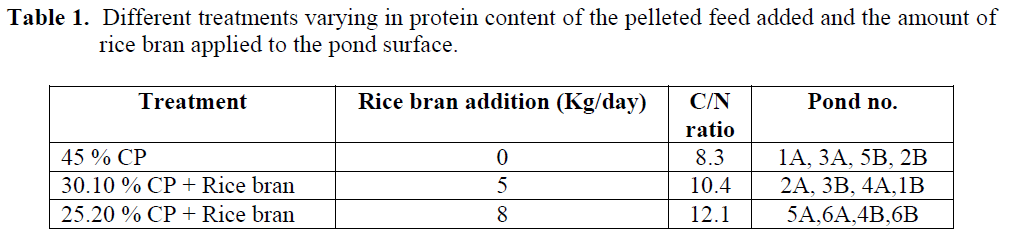
Table 1: Different treatments varying in protein content of the pelleted feed added and the amount of rice bran applied to the pond surface.
Figure 1: Layout of SAFICO farm and ponds used in the biofloc experiment and treatment assignment.
The organic C/N ratios were 8.3 in the treatments 45 % CP without Rice bran and 10.4 and 12.1 in the treatments 30.10 % CP and 25.20 % CP with Rice bran, respectively. Feeding start-ed at 7 AM, 12 PM, 4PM and 8PM. Rice bran addition was applied on pond surface daily at 10 AM.
Feed preparation:
Feeds were prepared on-farm sites (Table 2, Figure 1) with ingredients purchased from local an-imal feed supplier in Alexandria, Egypt. Proxi-mate analysis of the feeds was analyzed in Ani-mal Feed Research Institute, Regional Centre for Food and Feed, Agricultural Research center, Gi-za, Egypt.
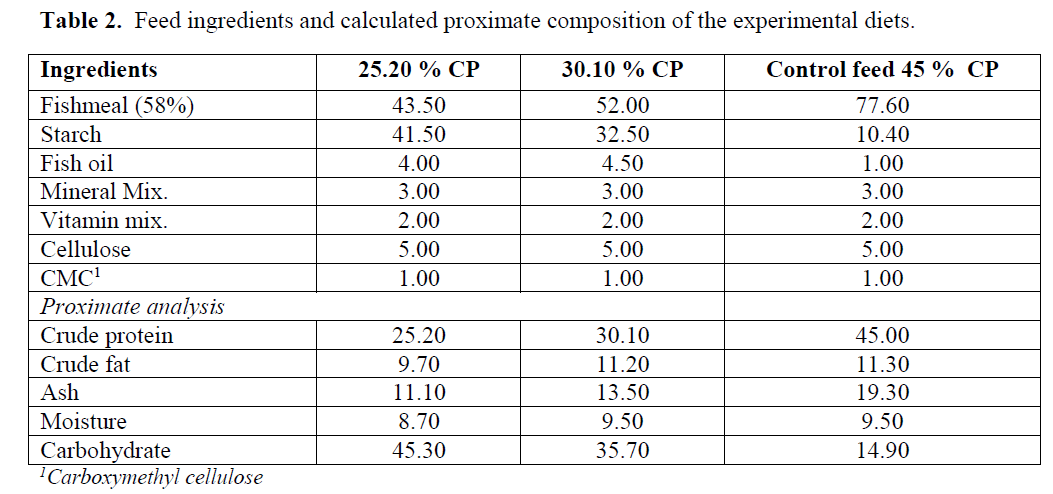
Table 2: Feed ingredients and calculated proximate composition of the experimental diets.
Sampling:
The impact of varying treatments was moni-tored by assessing Survival (SR %), Food Con-version Ratio (FCR), and Specific Growth Rate (SGR). Sampling was carried out every 7 days, to measure the average weight of the shrimp. Sur-vival and growth were calculated based on the following formulas:
Survival (SR %) = (Nf/Ni) × 100%
Where: Ni = initial number of shrimp stocked;
Nf= final number of shrimp harvested
FCR: Total feed used/weight gain of the sampled shrimp;
Growth rate (g/week) were calculated for each sampling interval by the formula G = (Wf _ Wi)/T, where G = growth rate in g/week, Wf = mean final weight (g), Wi = mean initial weight (g) and T = time (weeks).
Physico-Chemical Analysis:
The biofloc samples were analyzed for for Kjeldahl nitrogen (Kj-N), total ammonia nitrogen (TAN) total suspended solids (TSS) and volatile suspended solids (VSS). These properties were determined following APHA (1998). The differ-ence between Kjeldahl-N and TAN was used to calculate the protein content of the bioflocs by multiplying the organic nitrogen content by 6.25. The ash content was determined using TSS and VSS values. Lipids were extracted according to Folch et al. (1957), using the modification of Ways and Hanahan (1964). Protein, lipid and ash content were expressed as percentage of the dry weight (% DW) of the bioflocs. The total carbo-hydrate was calculated according to the following formula: carbohydrate (% DW) = 100 – (crude protein (% DW) + lipid (% DW) +ash (% DW)) (Manush et al., 2005) (Table 14). Fatty acid me-thyl esters (FAME) were prepared by transesteri-fication for gas chromatography according to Coutteau and Sorgeloos (1995) and identified by a gas chromatograph equipped with temperature programmable on-column injector.
Water quality:
Water quality in the culture systems was mon-itored daily for dissolved oxygen (DO), salinity, and temperature using a YSI 85m (YSI Inc., Ohio, US). Nitrate, nitrite, pH, and total ammonia nitrogen (TAN) were measured weekly using methods designed for seawater samples (Spotte, 1979). More detail for nitrate, nitrite, and TAN procedures can be found in Strickland and Par-sons (1972), Mullin and Riley (1955), and Solor-zano (1969), respectively.
Statistical Analysis:
Statistical analysis was performed using SPlus v 8.0 for Windows (Insightful Corp.). One- way ANOVA followed by Tukey’s test was employed to test the significant differences between treat-ments. Significance different was considered when P-value <0.05. A paired Student’s test was used for analyzing significant changes between two treatments with carbon source (when P<0.05).
Results and Discussion
Biofloc as a sustainable alternative to fish-meal in shrimp feed:
The biofloc experiment carried out in the growout ponds using cheap carbon source with the aim of developing least cost feed technology for sustainable shrimp farming in Egypt. Shrimp showed a similar growth with all diets. The shrimp were fed diets with low Fishmeal content had a slightly better growth and FCR, and the re-sults were statistically different (Table 3; P<0.05).
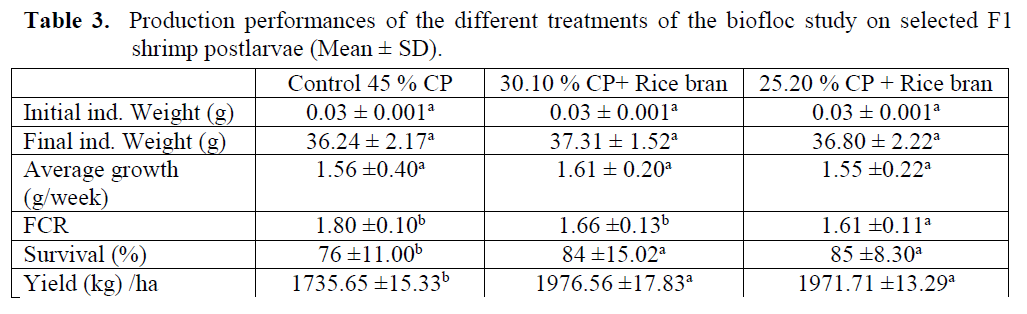
Table 3: Production performances of the different treatments of the biofloc study on selected F1 shrimp postlarvae (Mean ± SD).
The aim was to reduce the cost of feeds used to produce marine shrimp and reduction the utili-zation of marine proteins (fishmeal, squid meal, and shrimp meal) in the feeds and use the bio-flocs to recycle waste protein and nitrogen in the shrimp ponds to complement the pelleted feeds with live organisms. Two feeds were formulated and pressed on a 2 mm die and a reference diet with 45 % CP (Table 3) available locally and commercially for feeding marine fishes. The shrimp trial lasted for 150 days. Biofloc shrimp system yields high density production, low FCR’s, and superior survival rates using strong aeration and high quality probiotics and vitamins & premixes mixed with the carbon source. The biochemical composition of the bioflocs is shown in Table (4). Protein content was high in the 25.20 % CP + rice bran bioflocs, being 20 ± 7 % DW, while 16 ±5 in the 30.10 % CP+ rice bran. Crude lipid was also high in the 25.20 % CP + rice bran bioflocs 22 ±4 % DW. High ash con-tent was noted in the 25.20 % CP + rice bran bio-flocs, up to 4 ±3 % DW. The carbohydrate con-tent of the bioflocs was high in the 30.10 % CP+ rice bran treatment 61 ± 5% DW.
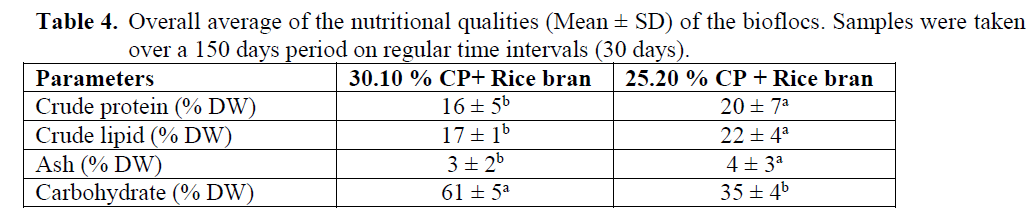
Table 4: Overall average of the nutritional qualities (Mean ± SD) of the bioflocs. Samples were taken over a 150 days period on regular time intervals (30 days).
The composition of the bioflocs of the two treatments (Table 4) didn’t vary significantly be-tween sampling dates; therefore mean values are discussed here. Bioflocs grown on 25.20 % CP + rice bran have a high protein content 20 ±7 DW. This microbial protein can serve as an additional high value feed for fish or shrimp, recycling the non-utilized fraction of the added conventional feed (Avnimelech et al., 1989). Protein levels in conventional feeds generally average 20-40% on the DW (Craig and Helfrich, 2002). Proteins are required in the diet to provide essential amino ac-ids. Fats are high-energy nutrients that can be uti-lized to partially substitute for protein in aquacul-ture feeds (Craig and Helfrich, 2002). Fats supply about twice the energy as proteins and carbohy-drates. Lipids typically comprise about 15% on the DW of fish diets, supplying essential fatty ac-ids. In this bioflocs experiment about 22 ±4 % fat on the DW was measured in the 25.20 % CP + rice bran treatment, 17 ±1 % DW in the 30.10 % CP+ rice bran.
The measured lipid content of all two types of bioflocs is high. The advantage is that the bio-flocs, when used as feed, will not need to be sup-plemented with a fat-containing material, since diets deficient in fats lead to lower growth and poor food conversion efficiency in fish (Tacon, 1990). High energy diets are widely used in salmon and trout farming, given the significant benefits of high levels of non-protein energy on improved protein retention and lower nitrogen extraction (Cho et al., 1994). High dietary fat can lead to an increase of the whole body fat con-tent. This increase in body lipid is mainly due to an increase in lipid content of liver and digestive tract, and not of the muscle tissue (Boujard et al., 2004). The ash content of the flocs in the 30% CP+ rice bran treatment was low 3 ± 2 % on the DW.
The PUFAs content is presented in Table (5). The total n-3 PUFAs of the bio-flocs produced from 25.20 % CP + Rice bran and 30.10 % CP+ Rice bran treatments was not significantly differ-ent from each other and fall in the range of 0.6 – 0.8 mg/g DW. With respect to the total n-6 PUFAs, the bio-flocs grown with 25.20 % CP + Rice bran treatment had a higher level than those grown with 30.10 % CP+ Rice bran treatment (Table 5). Regarding the composition of the PUFAs in the bio-flocs, the bio-flocs with 25.20 % CP + Rice bran treatment contained signifi-cantly more LNA (18:3(n-3)) than that with 30.10 % CP+ Rice bran treatment and the oppo-site for LA (18:2 (n-6)). In particular bio-flocs with 25.20 % CP + Rice bran treatment had a to-tal n-6 PUFAs.
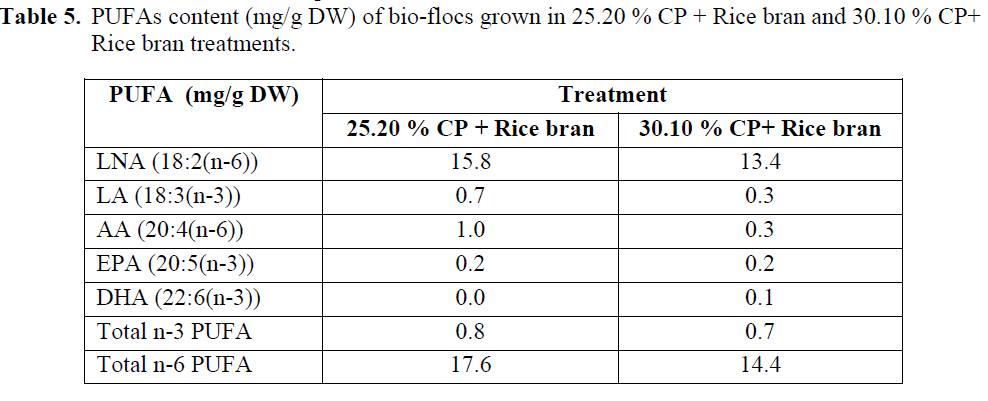
Table 5: PUFAs content (mg/g DW) of bio-flocs grown in 25.20 % CP + Rice bran and 30.10 % CP+ Rice bran treatments.
The bio-flocs were also rich in 16:0, 16:1n-7 and 18:1n-7 fatty acids which were similar to that reported for bacterial-based microbial communi-ties form biological phosphate removal systems (Liu et al., 2000; Izquierdo et al., 2006). A study carried out by Izquierdo et al. (2006) showed that the bio-flocs collected from a shrimp tank, where the shrimp were fed with a diet containing fish oil, contained a higher total n-3, n-6 and n-9 PUFAs than those fed with a feed that did not contain fish oil. Certik and Shimizu (1999) point-ed out that besides de novo synthesis of fatty ac-ids from glucose, the microbial fatty acid biosyn-thesis can be also carried out by the incorporation of exogenous fatty acids directly into lipid struc-tures followed by desaturation and elongation of lipid sources. This suggested that the fatty acid profile of the bio-flocs is affected by the dietary lipid composition. In this experiment the total n-3 PUFAs ranged from 0.6 to 0.8 mg/g DW whereas in the other study, where fish oil was included in the shrimp diet (Izquierdo et al., 2006). On the other hand, the total n-6 PUFAs content of the bio-flocs in this experiment, particularly with 25.20 % CP + Rice bran.
Complete diets are advised to contain less than 8.5% on the DW ash (Craig and Helfrich, 2002). High ash content lowers the digestibility of other ingredients in the diet resulting in poor growth of the fish. In the acetate bioflocs we not-ed higher ash content, i.e. 20% on the DW. This can decrease the digestibility of the acetate bio-flocs. The ash content of the glucose and starch bioflocs was lower than 8.5% on the DW. Carbo-hydrates are the most economical and inexpen-sive sources of energy for fish diets (Craig and Helfrich, 2002). Yet most of the fish species have a poor ability to utilize carbohydrates and they only represent a minor source of energy for fish. The flocs grown on 25.20 % CP + rice bran showed low carbohydrate content 35 ± 4% on the DW. Besides that bioflocs are overall a possible good additional nutritious aquaculture feed, the acceptance by the cultured species will play a crucial role in the use of bioflocs technology in aquaculture. The beneficial influence of bioflocs on the water quality in aquaculture systems has been investigated, but not all fish will be able to utilize bioflocs. Experimental evidence however, showed that certain aquaculture species can ef-fectively utilize bioflocs (Crab et al., 2009; Crab et al., 2010). Besides herbivores, more general detritus and benthos feeders can thrive on bio-flocs.
Microscopic Observations of Observations of Bio-Floc:
Biofloc developed in the shrimp ponds com-posed of Macroaggregates –diatoms, macroalgae (Bacterial filament, Chroococcus – cyanobacte-rium, – green alga), fecal pellets, exoskeleton, remains of dead organisms, bacteria, protest and invertebrates (Figure 2). It is unknown but cer-tainly doubtful if the same results would have been obtained in clear water without bioflocs. Research in the past has proven that the presence of bioflocs can increase growth and decrease FCR which means that shrimp can benefit from the nutritional quality of bioflocs. A good bal-anced feed can be produced without the utiliza-tion of marine proteins, as long as digestible pro-tein sources are used and amino acids are bal-anced. The diet without marine proteins is about 3500 L.E per tonne using cheaper raw materials; compared to 6000- 10000 L.E per tonne for commercial shrimp feeds available locally. The combination of such a diet and utilization of a bi-ofloc system enables sustainable production of shrimp anywhere in Egypt. In addition to eco-nomic characteristics, the biofloc is environmen-tal friendly because no water exchange during farming, recycling nutrient feces via bioflocs and limited utilization of natural resources.
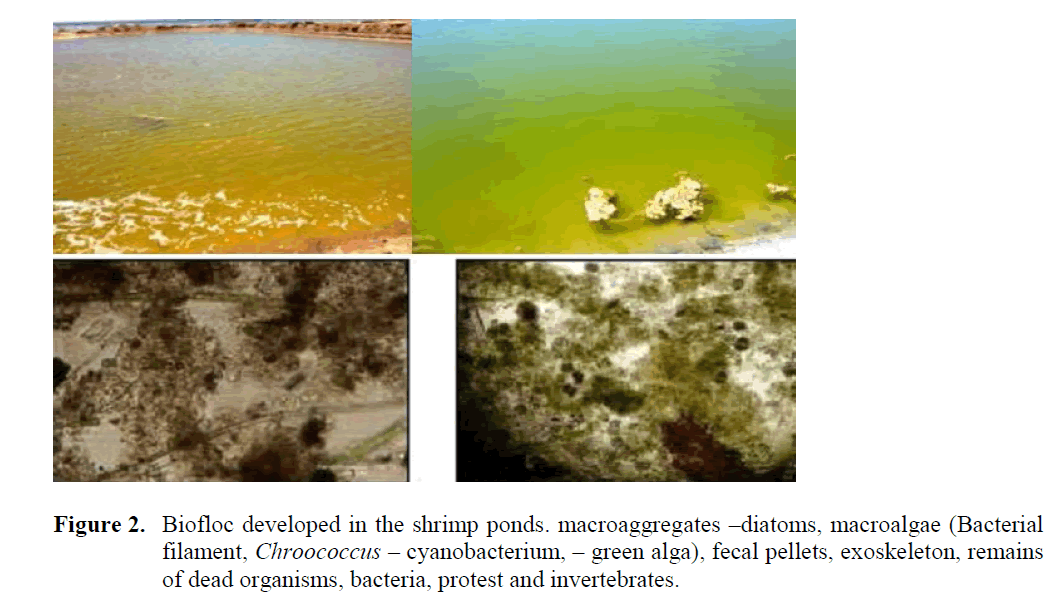
Figure 2: Biofloc developed in the shrimp ponds. macroaggregates –diatoms, macroalgae (Bacterial filament, Chroococcus – cyanobacterium, – green alga), fecal pellets, exoskeleton, remains of dead organisms, bacteria, protest and invertebrates. 337
Water quality:
The water quality results for pH, dissolved oxygen (DO), total ammonia nitrogen (TAN), ni-trite nitrogen (NO2--N) and nitrate nitrogen (NO2--N) were measured and are represented in Table (6). The temperature of the biofloc reactor water was around 25°C.
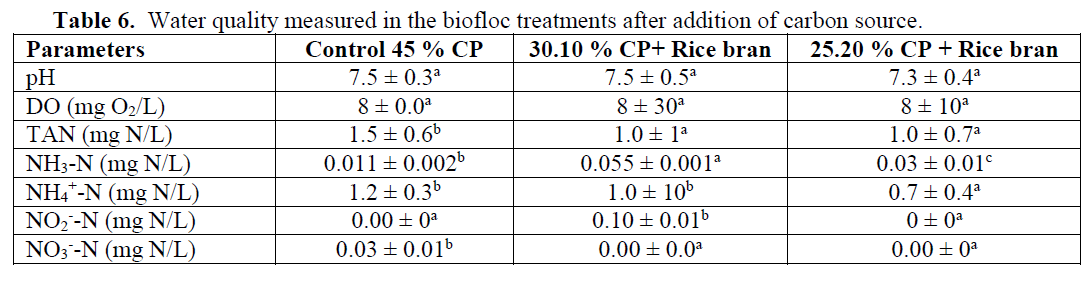
Table 6: Water quality measured in the biofloc treatments after addition of carbon source.
Samples were taken over a 150 days period on regular time intervals (30 days). Based on TAN concentrations, pH and temperature, concentra-tions of ammonia and ammonium were calculat-ed. The water quality parameters (Table 6) were for two treatments and control within acceptable range over the whole testing period of 150 days. Sampling dates didn’t significantly affect water quality parameters; hence mean values are dis-cussed here. The addition of carbon source re-duced water column TAN to 1.0 mg N/L, while nitrite-N and nitrate-N were both 0 mg N/L. One can calculate the concentration of ammonia (NH3) and ammonium (NH4+) derived from the TAN concentration and the pH. The calculated values indicate that for the carbon source treat-ments toxic levels are not exceeded. Ammonia is toxic to most commercial fish at concentrations above 1.5 mg N/L (Neori et al., 2004). Microbial flocs produced in this study could offer the shrimp industry a novel alternative feed and re-duction in the dependency on fish oil and fish-meal in feeding marine shrimp. In this study, mi-crobial flocs were produced in intensive shrimp greenhouses using wheat flour as a carbon source. Feed was applied at 5% of the total shrimp biomass in daily four rations. The nutri-tional quality of biofloc was appropriate for shrimp. There was significant difference (P<0.05) in shrimp growth/production between control and biofloc treatments of varying low protein levels.
Survival and abnormality were compared and no significant differences (P > 0.05) between BFT and control diet indicating no increased shrimp stress due to the presence of biofloc. Overall shrimp growth and production was good in terms of commercial feasibility. During the feeding experiment, it can be seen that the shrimp in all the biofloc treatments were able to consume the flocs (Figure 3). This was visually observed by the color of the digestive tract of the shrimp. The shrimp fed with control feed showed green-ish digestive tracts similar to the color of the feed whereas those fed with bioflocs revealed whitish and brownish digestive tracts, similar to the col-ors of the bioflocs.
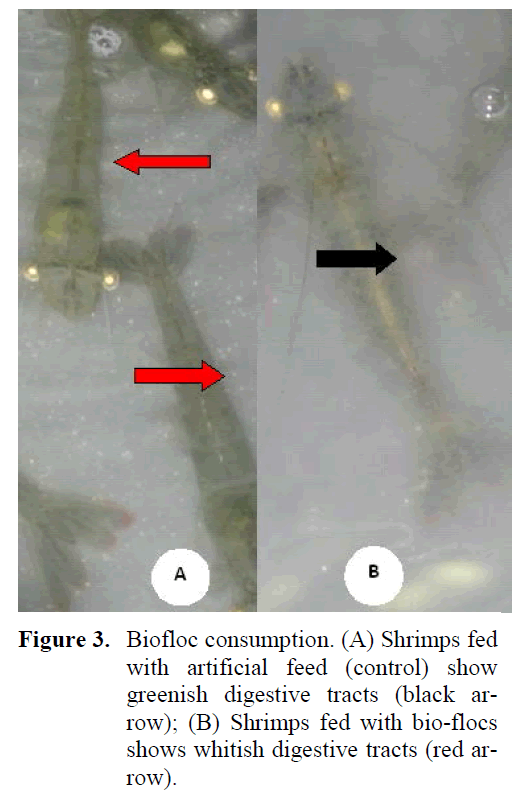
Figure 3: Biofloc consumption. (A) Shrimps fed with artificial feed (control) show greenish digestive tracts (black ar-row); (B) Shrimps fed with bio-flocs shows whitish digestive tracts (red ar-row).
There was no significant difference (P > 0.05) in survival between the control and all other treatments. The ABW of biofloc treatments was significantly (P < 0.05) higher than control and there was no significant difference (P > 0.05) among biofloc treatments. Based on visual obser- vation made during the experiment, the shrimps in control diet reduced feed intake which showed by sampling, checking feeding trays and by ob-serving the empty digestive tract. This can be due to several reasons such as the palatability of feed, stress due to disease infection or water quality deterioration. Also, Tacon (1987) found that the absence of feed attractant and low palatability may also have been the cause of less feed con-sumption in control diet. During the culture peri-od, the biofloc treatments showed good floc for-mation. Crab et al. (2007) pointed out that at moderate mixing rate as practiced in aquaculture system (1 – 10 W/m3), microbial cells in permea-ble aggregates grow better than single dispersed cells due to higher accessibility to the nutrients. Intense aeration on the other hand minimizes the advantage of growing in flocs and free cells show a higher nutrient uptake. There was no abnormal-ity or disease symptoms observed in control and biofloc treatments. Growth of shrimp as well as other aquatic organisms is mostly affected by wa-ter quality, culture systems (Tacon et al., 2002), nutrition (Chen et al, 2006) and health condition (Argue et al., 2002).
In terms of shrimp growth and feed utiliza-tion, it can be seen that shrimp growth was better in the low CP treatment, most likely due to the lower concentrations of toxic inorganic nitrogen species. In addition to a lower feed conversion ratio (FCR). According to Avnimelech (1999), the protein conversion ratio (PCR) was markedly reduced in the 20% protein treatment. The PCR in the conventional 30% protein feed treatment was 4.35–4.38, meaning that only 23% of the feed protein was recovered by the fish. The PCR in the low CP% was twice as high. The increased protein utilization is due to its recycling by the microorganisms. It may be said that the proteins are eaten by the fish twice, first in the feed and then harvested again as microbial proteins. It is possible that protein recycling and utilization can be further increased. Production results obtained in this study are within the range of commercial shrimp production in intensive production sys-tems. This study supports the theory that natural biota can provide a nitrogen source for shrimp, and that flocculated particles are likely to be a significant proportion of this nitrogen source. If this biofloc technology proved to be successful, it could offer the shrimp industry a new culture op-tion. A very significant further justification is the need to have alternative lower cost feeds replac-ing marine fish and shrimp meals. For these rea- sons, this study investigated if it would be possi-ble to produce microbial floc as a potential ingre-dient for reducing fishmeal in shrimp feeds.
Conclusion
The results of the present study showed that the use of biofloc represents a viable and more sustainable feed option due to cost, the manner in which it is generated, and the potential that it can decrease the pressure on wild fisheries by reduc-ing at least some of the demand for fishmeal. Al-so, the bioflocs technology is a sustainable tech-nique used in aquaculture to maintain good water quality through the development and control of dense heterotrophic microbial bioflocs by adding carbohydrate to the water. The results offered bi-ofloc as a sustainable and cost-effective alterna-tive source for costly and overexploited fishmeal in the feed of marine shrimp.
Acknowledgments
The author thanks all the members of the Fish farmers in “Shrimp and Fish International Com-pany (SAFICO)” group for the technical assis-tance.
55
References
- APHA., (1998). Standard Methods for the Exami-nation of the Water and Wastewater, 22nd edn. American Public Health Association, Washington, DC
- nArgue, B.J., Alcivar-Warren, A., (2000). Genetics and breeding applied to the Penaeid shrimp farming industry. In: Bullis, R.A., Pruder, G.D. Eds., Controlled and BiosecureProduc-tion Systems: Evolution and Integration of Chicken and Shrimp Models. Proceedings of a Special Session on Integration of Shrimp and Chicken Models, World Aquaculture So-ciety, Sydney, Australia, April 27–30, 1999. The Oceanic Institute, Waimanalo, HI, USA, pp. 29–54
- nAvnimelech, Y., (1999). Carbon/nitrogen ratio as a control element in aquaculture systems, Aquaculture, 176: 227-235. doi: 10.1016/S0044-8486(99)00085-X
- nAvnimelech, Y., (2005). Tilapia harvest microbial flocs in active suspension research pond. Global Aquaculture Advocate. October 2005
- nAvnimelech, Y., Mokady, S., Schroeder, G.L., (1989). Circulated ponds as efficient bioreactors for single-cell protein production, Israeli Journal of Aquaculture Bamidgeh, 41: 58-66
- nBoujard, T., Gélineau, A., Covíès, D., Corraze, G., Dutto, G., Gasset, E., Kaushik, S., (2004). Regulation of feed intake, growth, nutrient and energy utilization in European seabass (Dicentrarchuslabrax) fed high fat diets, Aquaculture, 231: 529-545. doi: 10.1016/j.aquaculture.2003.11.010
- nCertik, M., Shimizu, S., (1999). Biosynthesis and regulation of microbial polyunsaturated fatty acid production, Journal of Bioscience and Bioengineering, 87(1): 1-14. doi: 10.1016/S1389-1723(99)80001-2
- nChen, S.; Ling, J. and Blancheton, J.P., (2006). Nitrification kinetics of biofilm as affected by water quality factors, AquaucltureEngi-neering, 34: 179-197. doi: 10.1016/j.aquaeng.2005.09.004
- nCho, C.Y., Hynes, J.D., Wood, K.R., Yoshida, H.K., (1994). Development of high nutrient-dense, low-pollution diets and prediction of aquaculture wastes using biological ap-proaches, Aquaculture, 14: 293-305. doi: 10.1016/0044-8486(94)90403-0
- nCoutteau, P., Sorgeloos, P., (1995). Intercalibra-tion exercise on the qualitative and quantita-tive analysis of fatty acids in Artemia and marine samples used in mariculture. ICES Cooperative Research Report, N° 211, 30 pp
- nCrab R., Chielens B., Wille M., Bossier P. and Verstraete W., (2010). The effect of different carbon sources on the nutritional value of bi-oflocs, a feed for Macrobrachiumrosen-bergiipostlarvae, Aquaculture Research, 41: 559-567. doi: 10.1111/j.1365-2109.2009.02353.x
- nCrab R., Chielens B., Wille M., Bossier P. and Verstraete W., (2010). The effect of different carbon sources on the nutritional value of bi-oflocs, a feed for Macrobrachiumrosen-bergiipostlarvae, Aquaculture Research, 41: 559-567. doi: 10.1111/j.1365-2109.2009.02353.x
- nCrab, R., Avnimelech, Y., Defoirdt, T., Bossier, P., Verstraete, W., (2007). Nitrogen removal in aquaculture towards sustainable produc-tion, Aquaculture, 270: 1-14
- nCrab, R., Kochva, M., Verstraete, W., Avnimelech, Y., (2009). Bio-flocstechnolo-gy application in over-wintering of tilapia, Aquauclture Engineering, 40: 105-112. doi: 10.1016/j.aquaeng.2008.12.004
- nCraig, S., Helfrich, L.A., (2002). Understanding Fish Nutrition, Feeds and Feeding (Publica-tion 420-256). Virginia Cooperative Exten-sion, Yorktown (Virginia). 4 pp
- nFAO., (2009). Environmental impact assessment and monitoring in aquaculture. FAO Fisher-ies and Aquaculture Technical Paper. No. 527. Rome, FAO. 2009. 57p
- nFolch, J., Lees, M., Stanley, G.H.S., (1957). A simple method for the isolation and purifica-tion of total lipids from animal tissues, Jour-nal of Biological Chemistry, 226: 497-509
- nIzquierdo, M., Forster, I., Divakaran, S., Conquest, L., Decamp, O., Tacon, A., (2006). Effect of green and clear water and lipid source on survival, growth and biochemical composi-tion of Pacific white shrimp Litopenaeusvannamei, Aquaculture Nutrition, 12: 192-202. doi: 10.1111/j.1365-2095.2006.00385.x
- nKuhn, D.D.; Lawrence, A.L.; Boardman, G.D.; Patnaik, S., Marsh, L. and Flick Jr, G.J., (2010). Evaluation of two types of bioflocs derived from biological treatment of fish ef-fluent as feed ingredients for Pacific white shrimp, Litopenaeusvannamei, Aquaculture, 303: 28-33. doi: 10.1016/j.aquaculture.2010.03.001
- nLiu, W.T., Linning, K.D., Nakamura, K., Mino, T., Matsuo, T., Forney, L.J., (2000). Micro-bial community changes in biological phos-phate-removal systems on altering sludge phosphorus content, Environmental Microbi-ology, 146: 1099-1107
- nManush, S.M., Pal, A.K., Das, T., Mukherjee, S.C., (2005). Dietary high protein and vita-min C mitigate stress due to chelate claw ab-lation in Macrobrachiumrosenbergiimales, Comparative Biochemistry and Physiology A, 142: 10-18
- nMullin, J.D., Riley, J.P., (1955). The spectropho-tometric determination of nitrate in natural waters, with particular reference to sea-water, Analytical ChimicaActa, 12: 464-480. doi: 10.1016/S0003-2670(00)87865-4
- nNaylor, R.L., Hardy, R.W., Bureau, D.P., Chiu A., Eliott, M., Farrel, A.P., Forster, I., Gatlin, D.M., Goldburg, R.J., Hua, K., Nichols, P.D., (2009). Feeding aquaculture in an era of fi-nite resources, Proceeding of the National Academy of Sciences, 106: 15103-15110. doi: 10.1073/pnas.0905235106
- nNeori, A., Chopin, T., Troell, M., Buschmann, A.H., Kraemer, G.P., Halling, C., Shpigel, M., Yarish, C., (2004). Integrated aquacul-ture: rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture, Aquaculture, 231: 361-391.doi: 10.1016/j.aquaculture.2003.11.015
- nSchneider, O., Sereti, V., Eding, E.H., Verreth, J.A.J., (2005). Analysis of nutrient flows in integrated intensive aquaculture systems, Aquauclture Engineering, 32: 379-401. doi: 10.1016/j.aquaeng.2004.09.001
- nSolorzano, L., (1969). Determination of ammonia in natural waters by the phenolhypochlorite method, Limnology & Oceanography, 14: 799-801
- nSpotte, S., (1979). Fish and Invertebrate Culture: Water Management in Closed Systems, 2nd edition. Wiley, New York, New York, US
- nStrickland, J.D.H., Parsons, T.R., (1972). A Prac-tical Handbook of Seawater Analysis, 2nd edi-tion, Ottawa: Fisheries Research Board of Canada, Bulletin 167, 1968
- nTacon, A.G.J., (1987). The Nutrition and Feeding of Farmed Fish and Shrimp—A Training Manual. 1. The Essential Nutrients. Food and Agriculture Organization of the United Na-tions, GCP/RLA/075/ITA, Brazil, 117 pp
- nTacon, A.G.J., (1990). Standard methods for the nutrition and feeding of farmed fish and shrimp, Argent Laboratories Press, Washing-ton DC
- nTacon, A.G.J., Cody, J.J., Conquest, L.D., Di-vakaran, S., Forster, I.P., Decamp, O.E., (2002). Effect of culture system on the nutri-tion and growth performance of Pacific white shrimp Litopenaeusvannamei(Boone) fed different diets, Aquaculture Nutrition, 8(2): 121-137. doi: 10.1046/j.1365-2095.2002.00199.x
- nWays, P., Hanahan, D.J., (1964). Characterization and quantification of red cell lipids in normal man, Journal of Lipid Research, 5: 318-328.















