Keywords
Cyprinidae, Chalcalburnus mossulensis, nucleolar organizer region (NOR)
Introduction
Chromosomes carry ribosome genes coded for rRNA. The region(s) where these genes are copied in large numbers are called nucleolar organizer regions (NORs). NOR, which can be stained specifically, refracts light and due to this property, it can be seen very clearly. Structure, number and morphology of NOR may be specific to populations, species and subspecies. Thanks to this quality, NOR is used frequently to compare variations, as well as to identify and explain specifications. Changes in chromosome number and structure can alter the number and structure of NOR. Robertsonian translocations may cause losses of NOR. Species which have limited gene exchange due to geographical isolation have elevated karyotype and NOR variety. Therefore, different karyotypes are found even in small, but isolated populations of these species. Use of NORs in explaining kinships depends to a large extent on the uniformity of this characteristic and on the degree of variety within a taxon (Gülkaç, 1989; Küçükdumlu, 1995).
In Cyprinidae species, NOR can be found in different areas of different chromosomes (at the end of the short arms of submetacentric (SM) chromosome, on the short arms of subtelocentric (ST) chromosome, on the whole short arm of ST chromosome, on the long arms of subtelo-acrocentric (ST-A) chromosome, etc) (Gold et al., 1993; Pandas et al., 1993; Castro et al., 1996).
Chalcalburnus mossulensis lives only in the Eastern and Southeastern Anatolian Regions of Turkey, and particularly in the Tigris and the Euphrates River Systems. The present study aimed to analyze NOR in C. mossulensis, of the Cyprinidae family, living in Karakaya Dam Lake (Malatya, Turkey) in order to demonstrate similarities and differences within the species and between populations.
Materials and Methods
Nineteen fish specimens were caught of Karakaya Dam Lake (38º 27' 23.06" N, 38º 32' 17.47" E, 38º 28' 47.24" N, 38º 25' 55.24" E and 38º 29' 27.46" N, 38º 20' 23.82" E). The fish were carried to the laboratory in air conditioned barrels and kept in the aquarium for one week to relieve them of stress. For the karyological analysis of fish “air drying” method of Collares-Pereira (1992) and for NOR staining “a-1 step” method of Howell and Black (1980) were used. To this end, metaphase preparations were added to 70 microliter colloidal developer solution and 140 microliter aqueous silver nitrate solution. After the cover glass was mounted on the slide, it was put into the drying oven heated to 70°C. When their color turned to gold brown, preparations were taken out of the oven and washed with water. One to two ml of sodium thiosulfate solution was added on them. After several minutes of waiting, they were washed with water again. The preparations were stained for five minutes in giemsa solution, passed through xylene and acetone baths and closed with entellan. Preparations were screened for NOR analysis to guarantee the reliability of NOR numbers. Appropriate metaphases were photographed and chromosomes bearing NOR were marked and defined. The chromosomes were classified as suggested by Levan et al. (1964). Statistical analysis were evaluated by a one way analysis of variance, and the differences were considered significant if the P value was less than 0.05 by Tukey’s multiple comparison test. The specimens analyzed are deposited in the Cytogenetics Laboratory of the Department of Biology, Faculty of Science and Arts, University of Ahi Evran, 40200 K?r?ehir, Turkey, M. Gaffaroglu (M.G. 19).
Results and Discussion
In the study, number of diploid chromosomes was found 2n=50, of which six pairs were metacentric (M), eight pairs SM, five pairs ST, and six pairs A, and number of arms (NF) was found 88. NORs were identified in two pairs of SM chromosomes, one of which was large and the other medium-sized. NORs were located terminally on the longer arms of one pair of SM chromosomes, and terminally on the short arms of a pair of SM chromosomes (Figure 1). The data obtained were compared and contrasted with previous NOR studies.
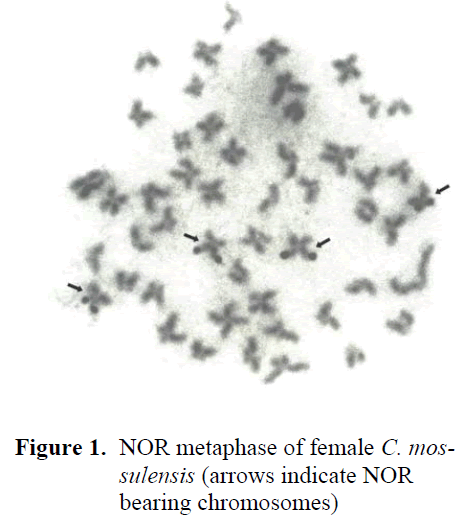
Figure 1. NOR metaphase of female C. mossulensis (arrows indicate NOR bearing chromosomes)
NORs can be perfect markers to display wide chromosomal polymorphism within and between species in many groups of fish. This variety may affect NOR number, its localization on the chromosome, size and active numbers in each genome (Ozouf-Costaz, 1992). Previous NOR studies showed variations between species, within species, and even between individuals (Galetti et al., 1984; Gold et al., 1993; Castro et al., 1996). NORs on different homologous chromosomes may be of different sizes. Some fish may even show a difference of up to almost two times in size between NORs found on the same homologous chromosome. It has been reported that this extent of variety between NORs may be attributed to number of sistrons and differences in transcriptional activity (Galetti et al., 1984).
NOR was found on a pair of chromosomes of Pteronotropis hubbsi (Amemiya and Gold, 1990a), Dionda episcopa, Ericymba buccata (Amemiya and Gold, 1990b), Leuciscus borysthenicus (Rab et al., 1996), Raiamas steindachneri (Rab et al., 2000), Labeo rohita, L. calbasu, L. bata (Gold et al., 1979) and on two pairs of chromosomes of Hybopsis aestivalis, Nocomis leptocephalus (Amemiya et al., 1988), Pimephales signipinnis, P. welaka, (Amemiya and Gold, 1990a), Leporinus obtusidens, Leporellus vittatus, Schizodon nasutus (Galetti et al., 1984), Champsocephalus gunnari (Ozouf-Costaz et al., 1996), Silurus glanis ve Barbus cyclolepis (Rab et al., 1996), as was the case in our study. In Campostoma anomalum (Amemiya and Gold, 1988) and Notropis roseipinnis (Amemiya and Gold, 1990b) NOR was found on three pairs of chromosomes, and in Aristichthys nobilis and Hypophthalmichthys molitrix (Almeida-Toledo et al., 1995) on four pairs of chromosomes.
Although NOR is generally seen at the end of short arms of ST (Sola et al., 1993; Rab et al., 1995) and SM (Rab et al., 1996) chromosomes, sometimes it may also be seen at the end of long arms of SM and ST chromosomes, on the arms of M (Galetti et al., 1984) and acrocentric (A) (Amemiya and Gold, 1990b chromosomes, as well as between telomeres and centromeres, and even adjacent to centromere (Jankun et al., 1998). NOR can also be found on sex chromosomes (Bertollo and Cavallaro, 1992), though very rarely. NOR was reported to be found terminally on the short arms of a pair of SM and a pair of ST chromosomes in N. ardens species (Gold and Amemiya, 1986), terminally on the short arms of a pair of medium-sized SM chromosome of Cobitis taenia (Boron, 1995), terminally on the short arm of a medium-sized SM chromosome and terminally on the short arm of a large STA chromosome in Pimephales signipinnis and P. welaka (Amemiya and Gold, 1990a), terminally on the short arms of two SM chromosomes of Silurus glanis (Rab et al., 1991), on an M-SM chromosome of Leporinus lacustrite, terminally on two SM chromosomes of L. elongates (Galetti et al., 1984), and terminally on the arms of a pair of M chromosomes and terminally on the long arms of a pair M chromosomes of Huso huso (Fontana et al., 1998). In our study, NOR was found on SM chromosomes.
NORs were reported to be small in some samples of Oedalechilus labeo species, but large in others (Rossi et al., 2000). NOR size was found heteromorphic at about the rate of 50% in Champsocephalus gunnari (Ozouf- Costaz, 1996). Similarly, heteromorphism was found in NOR size within species of Notropis buchanani, N. maculatus, Pteronotropis hubbsi, Pimephales signipinnis and P. welaka (Amemiya and Gold, 1990b). Capros aper was reported to show NOR size variations, which were attributed to duplication by the researcher (Rossi et al., 2000). In our examinations, there were no differences within a species in terms of NOR size and number. Furthermore, no difference in NOR size was found between the homologous and non-homologous chromosomes and their arms. In our study, one of the NORs was located terminally on the long arms of a pair of SM chromosomes, and the other was located terminally on the short arms of an SM chromosome pair. As indicated above, NOR is found on the same arms of the chromosomes; and when there are two pairs of chromosomes bearing NOR, these are located at the end of either long or short arms. However, sometimes NOR can be observed on both long and short arms, as was the case in our study. In our NOR screening in silver-stained preparations, although four (two pairs of) chromosomes bearing NOR were found in 68% of metaphases, two or three chromosomes bearing NOR were observed in 14%. Besides, there was no NOR in 18% of the metaphases in preparations. This may have been resulted from various reasons, such as insufficient NOR size, declined nucleolar activity in cells, errors in staining technique or failure of NORs to be stained. There is no variety within a species, within a population, between sexes and between individuals in terms of NOR (NOR number, localization and structure). There was no significant difference in chromosomal nucleolar organizer region phenotypes and in number of nucleolar organizer regions among localities.
In karyotype, NOR and other banding studies some researchers may obtain different results, and sometimes even can not obtain the desired result, due to the fact that fish chromosomes are too small and too many, and due to the reasons associated with the methods used. Factors like geographical differences, chromosome staining and banding methods may produce different results.
1615
References
- Almeida Toledo, L.F., Verri Bigoni, A.P., Bernardino, G., Almeida Toledo, F.S. (1995). Chromosomal location of NORs and C bands in F1 hybrids of bighead carp and silver reared in Brazil, Aquaculture, 135: 277-284.
- Amemiya, C.T., Gold, J.R. (1990a). Chromosomal NOR Phenotypes of Seven Species of North American Cyprinidae, with Comments on Cytosystematic relationships of the Notropis volucellus Species group, Opsopoeodus emiliae, and the Genus Pteronotropis, Copeia, 1: 68-78.
- Amemiya, C.T., Gold, J.R. (1990b). Cytogenetic studies in the North American minnows (Cyprinidae) Hereditas, 112: 231-247.
- Bertollo, L.A.C., Cavallaro, Z.I. (1992). A highly differentiated ZZ/ZW sex chromosome system in a Characidae fish, Triportheus guentheri, Cytogenetics and Cell Genetics, 60: 60-63.
- Boron, A. (1995). Chromosome banding studies of spined loach Cobitis taenia (L.), Cytobios, 81: 97-102.
- Castro, J., Vinas, A., Sanchez, L., Martinez, P. (1996).Characterization of an atypical NOR site polymorphism in brown trout (Salmo trutta) with Ag- and CMA3- staining, and flourescent in situ hybridization, Cytogenetics and Cell Genetics, 75: 234-239.
- Collares-Pereira, M.J. (1992). First Internatioal Workshop on Fish Cytogenetic Techniques, Concarneau, France, 14-24 September.
- Fontana, F., Tagliavini, J., Congui, L., Lanfredi, M., Chicca, M., Laurente, C., Rossi, R., (1998). Karyotipic characterization of the great sturgeon, Huso huso, by multiple staining techniques and flourescent in situ hybridization, Marine Biology, 132: 495-501.
- Galetti, P.M., Foresti, F., Bertollo, L.A.C., Moreria, F.O. (1984). Characterization of Eight Species of Anostomidae (Cypriniformes) Fish on the Basis of the Nucleolar Organizing Region, Caryologia, 37: 401-406.
- Gold, J.R., Amemiya, C.T. (1986). Cytogenetic Studies in North American minnows (Cyprinidae) XII. Patterns of Chromosomal Nucleolus Organizer Region Variation Among 14 Species, Canadian Journal of Zoology, 64: 1869-1877.
- Gold, J.R., Barat, A., Lakra, W.S. (1993). Localization of nucleolar organizer regions in Labeo (Cyprinidae), La Kromozoma II, 70: 2381-2384.
- Gold, J.R., Whitlock, C.W., Karel, W.J., Barlow, J.A. (1979). Cytogenetic studies in North American minnows (Cyprinidae) 6 karyotypes of thirteen species in the genus Notropis, Cytologia, 44: 457-466.
- Gülkaç, M.D.,Yüksel, E. (1989). Malatya Yöresi Kör Fareleri (Rodentia: Spalacidae) üzerine sitogenetik bir inceleme, Doga Türk Biyoloji Dergisi, 13: 63-71.
- Howell, W.M., Black, D.A. (1980). Controlled Silver Staining of Nucleolus Organizer Regions with a Protective Colloidal Developer: a-1 Step Method, Experientia, 36: 1014-1015.
- Jankun, M., Woznicki, P., Dajnowicz, G., Demska-Zakes, K., Luczynski, M.J. (1998). Heterochromatin and NOR location in northern pike (Esox lucius), Aquatic Sciences, 60: 17-21.
- Küçükdumlu, I. (1995). Spalax leucodon ve Spalax ehrenbergi’de Nükleolus Organizatör Bölgenin Tespiti ve Evolusyoner Durumu, Yüksek Lisans Tezi, Danisman Gülkaç M.D., Inönü Üniversitesi, Fen Bilimleri Enstitüsü, Malatya
- Levan, A., Fredga, K., Sandberg, A.A. (1964). Nomenclature for Centromeric Position on Chromosomes, Hereditas, 52: 201-220.
- Ozouf-Costaz, C., Foresti, F. (1992). Fish Cytogenetic research: advances, applications and perspectives, Nedherland Journal of Zoology, 42: 277-290.
- Rab, P., Karakousis, Y., Rabova, M., Economidis, P.S. (1996). Banded karyotype of the Cyprinid fish Leuciscus borysthenicus, Ichthyological Research, 463-468.
- Rab, P., Machordom, A., Rabova, M., Doadrio, I. (2000). Karyotype of African bariliine fish Raimas steindachneri (Osteichthyes, Cyprinidae), Folia Zoology, 49: 75-80.
- Rab, P., Mayr, B., Roth, P. (1991). Chromosome banding study of European Catfish, Silurus glanis (Pisces, Siluridae), Chromosoma, 83: 153-157.
- Rossi, A.R., Gornung, E., Crosetti, D., Innocentti, S., Sola L. (2000). Cytogenetic analysis of Oedalechilus labeo (Pisces: Muglidae), with a report of NOR variability, Marine Biology, 136: 159-162.
- Sola, L., Bressanello, S., Rossi, A.R., Iaselli, V., Crosetti, D., Cataudella, S. (1993). A karyotype analysis of the genus Dicentrarchus by different staining techniques, Journal of Fish Biology, 43: 329-337.







