Keywords
Sexual dimorphism, growth, Black Sea turbot, Psetta maxima
Introduction
The turbot, Psetta maxima, is distributed from Norway along the European coast to the Mediterranean and Black Sea. The production of the turbot has been attempted since the 1970’s in some European countries due to its high commercial value, and in recent years the commercial culture of this species has benefited from improvements in larval rearing methods, and nutrition and husbandry practices in the ongrowing phase (Jones, 1973; Person-Le Ruyet and Noel, 1982; Burel et al., 1996; Mallekh et al., 1998; Irwin, 1999). However, large individual variations in growth performance have been observed in turbot (Rosenberg and Haugen, 1982; Imland et al. 1995) and there is a need to optimize production characteristics (Lavens and Remmerswaal, 1994).
Turbot exhibits sexually dimorphic growth. Males start to grow less than females as early as 8 months after hatching (Imsland et al. 1997), and this differential growth rate is maintained throughout the remainder of the production cycle, including sexual maturation. Maturing females can reach 1.8 kg in 20 months whereas the weight of males only reaches around 1 kg. Thus, as is practiced with other cultured species, the development of techniques for preferentially producing female turbot is necessary to optimize production of this species (Xu et al. 2008).
Reproductive status greatly affects individual growth rates as less energy is committed to somatic growth. The ability of a fish farmer to control the ratio of male to female fish or to produce single-sex stocks has significant economic implications and many positive benefits for the environment. By controlling the number of males to females, the fish farmer can reduce the number of broodstock necessary to obtain a given egg take or increase egg take by rearing mainly females. The sex showing the greatest growth potential can also be selected for; for example, the female turbot can grow to a much larger size than the male.
The main aim of this study was to validate all-female populations of turbot for commercial production, first by determining whether females grow at a faster rate than males during commercial cultivation and second by identifying the age and/or size at which sex linked growth divergence occurs.
Materials and Methods
Experimental trials were conducted at the Central Fisheries Research Institute (CFRI), Trabzon, Turkey. A total of 119 turbot (18 months old), 63 males weighing 582.5±112.50 g and 56 females weighing 541.8±123.85 g, used in this study were obtained from brood stock in the spawning season in 2001 at the CFRI. The experiment lasted 364 days, from 14 January 2003 to 14 January 2004.
Sex identification were done by using the desk light irradiation method described by Çiftçi et. al (2002). A total of 2 experimental groups were set up in replicates as follows: males and females, and replaced in the circular fiberglass indoor tanks of 3.14 m2 capacity, with an operating depth of 50 cm. The water flow rate was 15 l/min. The seawater used in the hatchery was pre-treated using pressurized sand filters and a UV sterilization system. Over the duration of the study, water quality parameters were maintained within safe limits. The water was aerated with two air stones at a moderate rate. Natural illumination and day-length were maintained in the tanks during the experimental period. Temperature (twice in a day), dissolved oxygen (DO) and pH values (weekly intervals) were measured. The specimens were hand-fed to satiation with frozen whiting twice a day during the light period and daily food consumption was recorded for each tank.
Specific growth rate (SGR), the daily feeding rate (FR), and food conversion ratio (FCR) were calculated as follows:
SGR=100[ln(Wt)-ln(W0)/t]
FR={(Σfk)/[t*( Wt+W0)/2]}*100
FCR=(Σfk)/(Wt-W0)+m;
where t: feeding days (day), W0: initial live weight of fish (g), Wt: final live weight of fish (g) and fk: dry weight of feed supplied by the group of fish at each feeding (g), m: weight of dead fish (g).
To calculate and monitor various growth parameters and predict a daily food ration, total length and weight of fish were taken individually before the start of the trial and then again every 3 months.
Data are represented as arithmetic means of individual weights ±SD. A Kolmogorov-Smirnov test indicated that the data were distributed normally (P >0.05) and homogeneity of variances was tested using the Levene’s F-test. A t-test was used to determine differences between replicates. One-way ANOVA was used to test for differences among experimental groups and was followed by Tukey’s multiple range tests when the differences were significant. Statistical analyses were performed with Statistica 7, Stat Soft. Inc.
Results and Discussion
Water quality was fairly constant during the experimental period. Seawater temperature was between 8.0 and 24.1 ºC (15.0 ±4.9). DO and pH values ranged within optimal limits and were 5.3-9.5 mg/l (7.1 ±0.9) and 7.5-8.5 (8.1 ±0.2), respectively. There were no significant differences among experimental treatments in terms of DO and pH values.
The mean body weight and length measured in 63 males and 56 females are shown in Table 1 and Table 2. There were no significant differences in weight and length between experimental groups at the beginning of the experiment. No significant differences were found between replicates of the groups over the course of the experiment and so data from replicates were pooled for each treatment prior to analysis. Throughout the trial, all food was consumed regardless of group, and all fish remained healthy with no mortalities.
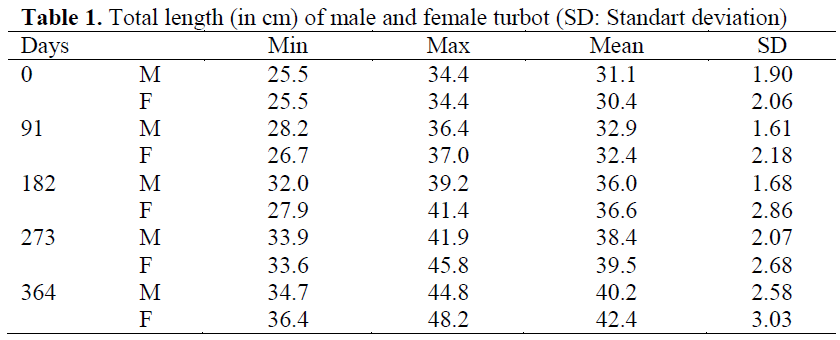
Table 1: Total length (in cm) of male and female turbot (SD: Standart deviation)
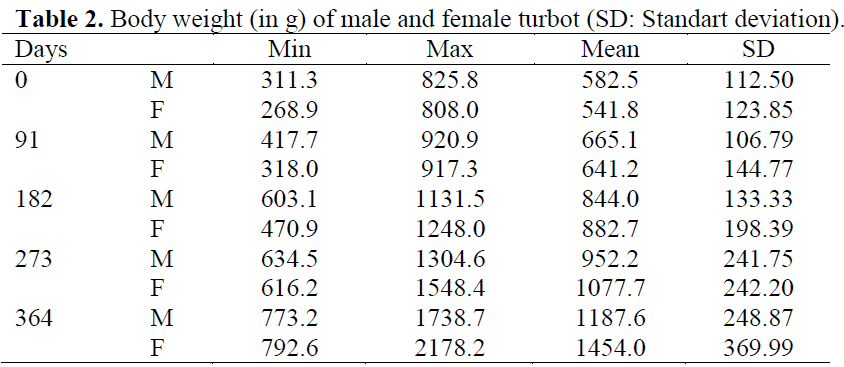
Table 2: Body weight (in g) of male and female turbot (SD: Standart deviation).
Growth and feeding data are displayed in Table 3. Over the duration of the study, SGRs ranged between 0.19 and 0.35% (mean 0.27%±0.039) for females, and between 0.13 and 0.26% (mean 0.20%±0.001) for males. The growth data clearly indicated the SGR, growth rate, mean growth and length gain values of females were significantly higher than those of males (Table 3; P < 0.05).
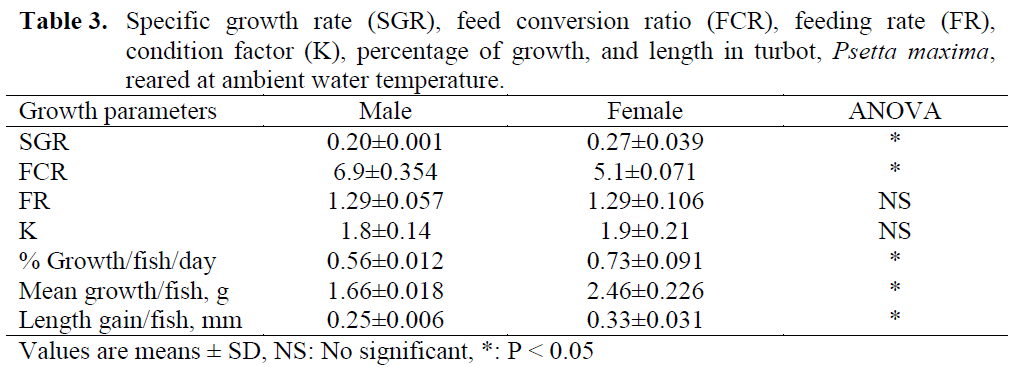
Table 3: Specific growth rate (SGR), feed conversion ratio (FCR), feeding rate (FR), condition factor (K), percentage of growth, and length in turbot, Psetta maxima, reared at ambient water temperature.
K values were checked at the beginning of the study and every three months intervals. Differences between sexes were not significant (Table 3).
At the end of the study, on day 364, although there was no difference between sexes on the FR, the FCR in females were significantly higher than males (P < 0.05).
In the present study, females grew better than males in accordance with earlier studies on turbot (Jones, 1974; Robert and Vianet, 1988; Déniel, 1990). Final weight of male was 82% of the mean final weight of female.
The turbot specimens used in this study were at 18 months post-hatch, and at this time we could positively identify the sex of individuals. The results showed no significant increase in female proportion, suggesting that growth divergence between the sexes had not taken place. Furthermore, the average size of males and females was approximately equal, as shown in Table 1 and Table 2. However, a large range in weight of individuals between sexes (1187.6-1454.0 g) was observed, even after 30 months the first sexual maturation had taken place, this may be due to sex-linked growth traits. Parker (1992) and Rypel (2007) stated that a divergence in body size between sexes could be related to different reproductive investments. Under culture conditions, the first sexual maturity in turbot takes place at an age of about 24 months, and the first maturity does not negatively affect growth, since by that time, the gonads are still not too big (Cal et al., 2006).
Lozán (1992) found that on average, females consumed 73% more food than similar-sized males, and that females had a significantly larger digestive tract than males. He concluded that because of these differences females would grow better than males. According to Imsland et al. (1997) there might be two explanations in growth differences between sexes; one possible explanation of the observed sexual growth divergence might be differences in food intake, and another possible explanation of growth differences are sex-related maturity differences influencing growth.
In this study, although there was no differences between sexes on the FR, the FCR in females were significantly higher than males (P < 0.05), and significant growth and size differences between sexes were found in 30 months after hatching (after first sexual maturation). It is possible that part of the observed growth differences seen in the present study originate from different digestive tract and sexual dimorphism.
Conclusion
In conclusion, turbot males grew less than females and significant growth differences between females and males occurred at the age of 30 months after hatching. Maturing females had the highest growth and reached the largest average total length and weight (42.4 cm and 1454.0 g in 30 months). Results from this study illustrate a clear value to all-female production for the commercial production of turbot. Additionally, this growth advantage substantiates the need to develop techniques for the production of all-female juveniles in the hatchery.
815
References
- Burel, C., Person-Le Ruyet, J., Gaumet, F., Le Roux, A., Sévère, A., Boeuf, G., (1996). Effects of temperature on growth and metabolism in juvenile turbot, Journal of Fish Biology, 49: 678-692. doi:10.1111/j.1095-8649.1996.tb00064.x
- Cal, R.M., Vidal, S., Gomez C., Alvarez-Bla´zquez, B., Marti´nez P., Piferrer, F., (2006). Growth and gonadal development in diploid and triploid turbot (Scophthalmus maximus), Aquaculture, 251: 99–108. doi:10.1016/j.aquaculture.2005.05.010
- Çiftçi, Y., Üstündag, C., Erteken, A., Özongun, M., Ceylan, B., Hasimoglu, A., Günes, E., Yoseda, K., Sakamoto, F., Nezaki, G., Shiro, H., (2002). Manual for the Seed Production of Turbot, Pesetta maxima in the Black Sea. Special Publication No. 2, Central Fisheries Research Institute, Ministry of Agriculture and Rural Affairs, Trabzon, Turkey and Japan Cooperation Agency, 80 pp.
- Déniel, C. (1990). Comparative study of growth of flatfishes on the west coast of Britanny, Journal of Fish Biology, 37: 149-166. doi:10.1111/j.1095-8649.1990.tb05936.x
- Imsland, A.K., Folkvord. A., Stefansson, S.O., (1995). Growth, oxygen consumption and activity of juvenile turbot (Scophthalmus maximus) reared under different temperature and and photoperiods, Netherlands Journal of Sea Research, 34: 149-159. doi:10.1016/0077-7579(95)90023-3
- Imsland, A.K., Folkvord, A., Grung, G.L., Stefansson, S.O., (1997). Sexual dimorphism in growth and maturation of turbot Scophthalmus maximus (Rafinesque, 1810), Aquaculture Research, 28: 101– 114. doi:10.1111/j.1365-2109.1997.tb01022.x
- Irwin, S., Halloran, J.O., FitzGerald, R.O., (1999). Stocking density, growth and growth variation in juvenile turbot, (Scophthalmus maximus (Rafinesque), Aquaculture, 178: 77-88. doi:10.1016/S0044-8486(99)00122-2
- Jones, A., (1973). Observations on the growth of turbot larvae, Scophthalmus maximus L., reared in the laboratory, Aquaculture, 2: 149-155. doi:10.1016/0044-8486(73)90142-7
- Jones, A., (1974). Sexual maturity, fecundity and growth of the turbot, Scophthalmus maximus L., Journal of the Marine Biological Association of the United Kingdom, 54: 109-125. doi:10.1017/S0025315400022104
- Lavens, P., Remmerswaal, R.A.M. (1994). Turbot Culture. Problems and Prospects, European Aquaculture Society, Ootende, Belgium, 358 pp.
- Lozán. J.L., (1992). Sexual differences in food intake, digestive tract size, and growth performance of the dab, Limanda limanda L, Netherlands Journal of Sea Research, 29: 223-227. doi:10.1016/0077-7579(92)90022-7
- Mallekh, R., Lagardre, J.P., Bgout Anras, M.L., Lafaye, J.Y., (1998). Variability in appetite of turbot, Scophthalmus maximus under intensive rearing conditions: the role of environmental factors, Aquaculture, 165: 123-138. doi:10.1016/S0044-8486(98) 00244-0
- Parker, G., (1992). The evolution of sexual size dimorphism in fish, Journal of Fish Biology 41: 1–20. doi:10.1111/j.1095-8649.1992.tb03864.x
- Person-Le Ruyet, J., Noël, T., (1982). Effects of moist pelleted food an the growth of hatchery reared turbot (Scophthalmus maximus L) juveniles, Journal of the World Mariculture Society, 13: 237-245.
- Robert, F., Vianet, R. 1988. Age and growth of Psetta maxima (Linné, 1758) and Scophthalmus rhombus (Linné, 1758) in the Golf of Lion (Mediterranean), Journal of Applied Ichthyology, 4: 111-120. doi:10.1111/j.1439-0426.1988.tb00550.x
- Rosenberg, A.A., and Haugen, A.S., (1982). Individual growth and size-selective mortality of larval turbot (Scophthalmus maximus) reared in enclosures. Marine Biology, 72: 73-77. doi:10.1007/BF00393950
- Rypel, A.L., (2007). Sexual Dimorphism in Growth of Freshwater Drum, Southeastern Naturalist, 6(2):333–342. doi:10.1656/1528-7092(2007)6[333:SDIGOF]2.0.CO;2
- Xu, J-H, You., F., Sun, W., Yan, B-Y., Zhang, P-J., (2008). Induction of diploid gynogenesis in turbot Scophthalmus maximus with left-eyed flounder Paralichthys olivaceus sperm, Aquaculture International, 16: 623–634. doi:10.1007/s10499-008-9173-y









