Keywords
Starter cultures; Nisin; Tambaqui; Colossoma macropomum; Fishburger; Conservation
Introduction
Fish is an excellent food source for humans because it provides valuable protein and vitamins, especially A and D (Roos et al., 2007). Of the total amount of fish processed in Brazil, a significant portion is not used due to a lack of knowledge or processing technologies. For example, fishburgers, stuffed products (such as sausages), fish paste, surimi, breaded fish, extracted oils, and other food forms can further exploit the nutritional potential of fish (Ogawa, 1999; Ta¸kaya, et al., 2003). Additionally, other fish-based products have begun to appear on the market, suggesting that consumers are developing an increased awareness of the nutritional qualities of fish (Gonçalves et al., 2009; Lourenço et al., 2010).Tambaqui (Colossoma macropomum) belongs to the Characidae family and the Colossoma genus. It is a native species of the Amazon that responds well to intensive farming; thus, it is the main native species available to farmers in northern Brazil. Because the tambaqui is omnivorous, it has a great capacity to adapt to various types of food available in nurseries. It is a rustic fish that tolerates low levels of dissolved oxygen in water, and its supply of fingerlings is great. It has good growth potential and high productivity. Due to these characteristics, this species has been the subject of various studies, with the aim of creating a database that can contribute to the development of best practices for cultivating the species (Araújo-Lima and Gomes, 2005; Chagas et al., 2007).
The biopreservation of food has been thoroughly studied and used to enhance food safety and extend its commercial life. This technique can be defined as the use of one or more organisms to control or inhibit other organisms. Biopreservation can involve living organisms, e.g., lactic acid bacteria (LAB), or can occur indirectly through certain agents they produce. Among these agents are bacteriocins, which are peptides or proteins with ribosomal synthesized antibacterial activity that do not damage the cells that produce them (Konings et al., 2000; Rosa et al., 2002; Jay et al., 2005; Nascimento et al., 2008).Bacteriocins are often confused with antibiotics. Because of this, and because of legal restrictions in the use of antibiotics in food, it is important to make a distinction between these. Antibiotics, unlike bacteriocin (synthesized ribosomes) are products of microorganisms’ secondary metabolism, especially that of fungi, and primarily have a clinical purpose (Chikindas et al., 2001; Martines et al., 2003; Jay et al., 2005; Gálvez et al., 2007).Starter cultures containing staphylococci species are widely used in Europe and Asia. Most often, these cultures are already associated with a strain of LAB, and the most common are Staphylococcus xylosus, Staphylococcus carnosus and Lactobacillus curvatus. Certain Staphylococci spp. strongly affect the flavor of the food in which they are employed (Olensen and Stahnke, 2004; Talon and Leroy, 2006).
Therefore, bacteriocins provide another resource for food preservation, especially when used with other barriers, as some bacteriocins are heat resistant, act on a broad spectrum of microorganisms, are inactivated by digestive proteases, act in acidic environments and, most importantly, are generally regarded as safe (GRAS) (Chikindas et al., 2001; Rosa et al., 2002; Jay et al., 2005; Thomas et al., 2005; Gálvez et al., 2007; Calo-Mata et al., 2008). LAB, when used with starter culture, further reinforces the safety of foods to which is applied (Talon and Leroy, 2006). The aim of this work was to study the effects of individual use of starter culture, nisin, and the combination starter culture-nisin on production and conservation of molded tambaqui (Colossoma macropomum) fishburguer type.
Material and Methods
Tambaqui (Colossoma macropomum) used in this study were supplied by the with the same genetic origin were used, raised in masonry tanks in the Experimental Station of Freshwater Pisciculture of the Federal Rural University of the Amazon (UFRA). Tambaqui were gutted and beheaded, and the bones and skin were removed in the laboratory (at 15º C). Fillets were washed and then minced with meat mixer before the addition of fish paste (80%), cassava flour (10%), salt (3%), black pepper (0,5%), garlic (1%), water (5,5%).
The base formulation was divided into four portions: an untreated control (C); one containing a starter culture (T) of Staphylococcus xylosus, Staphylococcus carnosus and Lactobacillus curvatus (Texel®, DANISCO - Master Sense, Jundiaí, SP); one containing nisin (N) (nisaplin ®, DANISCO - Master Sense, Jundiaí, SP); and a fourth containing a mix of equal parts of nisin and starter culture (NT). For each group, 5.625 kg of the product was prepared and shaped so that the formed product would weigh approximately 40 g. The products were vacuum-packed (FASTVAC F200, São Paulo) in 15 cm × 22 cm plastic (Flexpack, São Paulo) and kept refrigerated at an average temperature of 6 ± 1°C. The duration of this experiment was 35 days.
Before the starter culture was added to the formulations, it was reactivated in 200 ml of 30°C water for 30 minutes, as recommended by the supplier. Both the T (starter culture only) and NT (nisin and starter culture) products received 11.25 ml (1.125 g) of reactivated starter culture for each 5.625 kg of the product.
The nisin was diluted in distilled water with its pH previously adjusted to between 5 and 6 with phosphoric acid, so that its concentration was 200 mg/kg of the base paste.
Physical and chemical analysis
The physicochemical tests were performed according to AOAC (1997) and included pH, according to Brazil (1981); total volatile bases (TVB) (Brazil, 1997); water activity (aw) (Decagon Aqualab 3TE) and color (Minolta CR 310). Texture measurements were performed in a QTS Texture Analyzer - Brook Field, using a 5-mm diameter inox probe. There were five replications of each product. The test height was 134,34 mm and the rate of descent, 60 mm/min. Penetration of the probe was up to 3,5 mm (70% of the thickness). The raw material and the elaborated products were analyzed for moisture, fat, protein, ash, TVB, aw and color. The same tests, except for TVB, were performed on the manufactured products, which were also analyzed for texture. The fat, protein and ash contents of the products were analyzed only at Time 5 and there were three replicates per product.
Microbiological analysis
Both the raw materials and the manufactured products were analyzed for total and fecal coliform bacteria (MPN/g), Salmonella (25 g) and coagulase-positive staphylococci (CFU/g). The total plate counts of mesophilic bacterias (CFU/g) were performed on the raw materials, on the products containing nisin and on the control (without nisin and without starter culture), since previous analysis indicated the interference of starter culture in these products (T and NT). The total plate count of psycrophilic bacterias were performed on raw materials and on all elaborated products. The lactic acid bacteria (LAB) count was performed only on products that contained the starter culture (T and NT). All products were examined for mold and yeast used MRS agar. All tests were performed according to Silva et al. (2007) and Vanderzant and Splittstoesser (1992), with three replicates per product.
Statistical analysis
The data were subjected to analysis of variance (ANOVA) and Tukey tests using STATISTICA 5.0 (StatSoft) software. P values <0.05 were considered significant.
Discussion
Physicochemical analysis
The results of the characterization of raw materials at time 0 and manufactured products (lipids, proteins and ash) at time 5, are shown in Table 1.
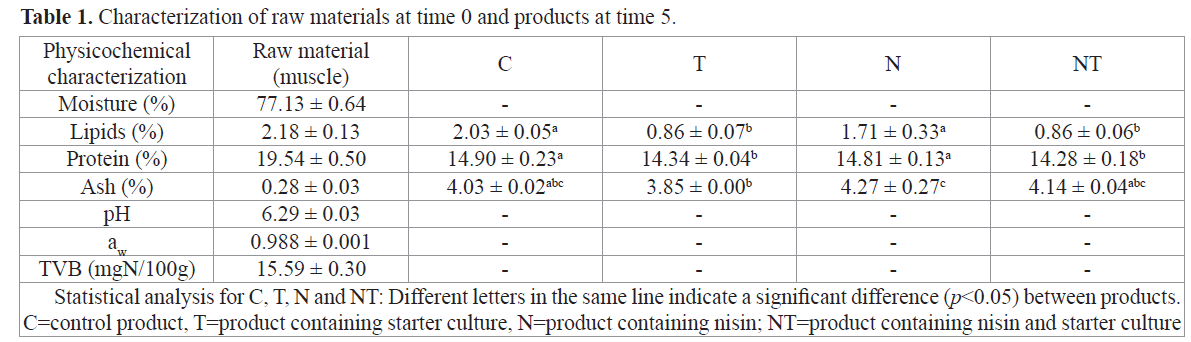
Table 1. Characterization of raw materials at time 0 and products at time 5.
The lipid content of the raw material (Table 1) was determined only for muscle. Arbeláez-Rojas et al. (2002) studied tambaqui reared in semiintensive and intensive regimes and found lipid levels of 2.41% and 1.40%, respectively, compared with 2.18% found in the raw material used in this study. They also emphasized that in this species; fat accumulates mainly around the viscera and varies little in the fillet throughout the year. The determination of lipid, proteins and ash on products, were held at time 5. The lipid content of the products was influenced by the presence of starter culture, with significant differences (p<0.05) between these products. This difference is explained by the presence of lipolytic agents produced by strains represented in the starter culture as esterases or lipases. Keneally et al. (1998) assessed the lipolytic activity of various strains of lactobacilli, pediococci, staphylococci and micrococci. Marked lipolytic activity was found in strains of Staphylococcus and Micrococcus, and Staphylococcus xylosus was the most active. The action of these agents is the first step towards oxidative degradation (Casaburi et al., 2008).The results showed that protein content of Products C and NT was statistically similar, and that this value was different from that of Products T and N. Because the temperature was controlled to remain at approximately 6°C, it is likely that the products that received the starter culture did not completely ferment, which could lead to marked proteolysis. Hu et al. (2008) observed similar behavior for other products with starter cultures. However, in their studies of golden carp sausages that underwent a fermentation process at 30°C for 48 hours, protein levels were lower after fermentation compared with controls, due to protein degradation.The results for ash content differed only between products T and N. Ash measurements conducted by Hu et al. (2008) in sausages that received starter culture presented no differences compared with controls. Table 2 shows the results of analytical determinations of moisture, pH and aw over 35 days.
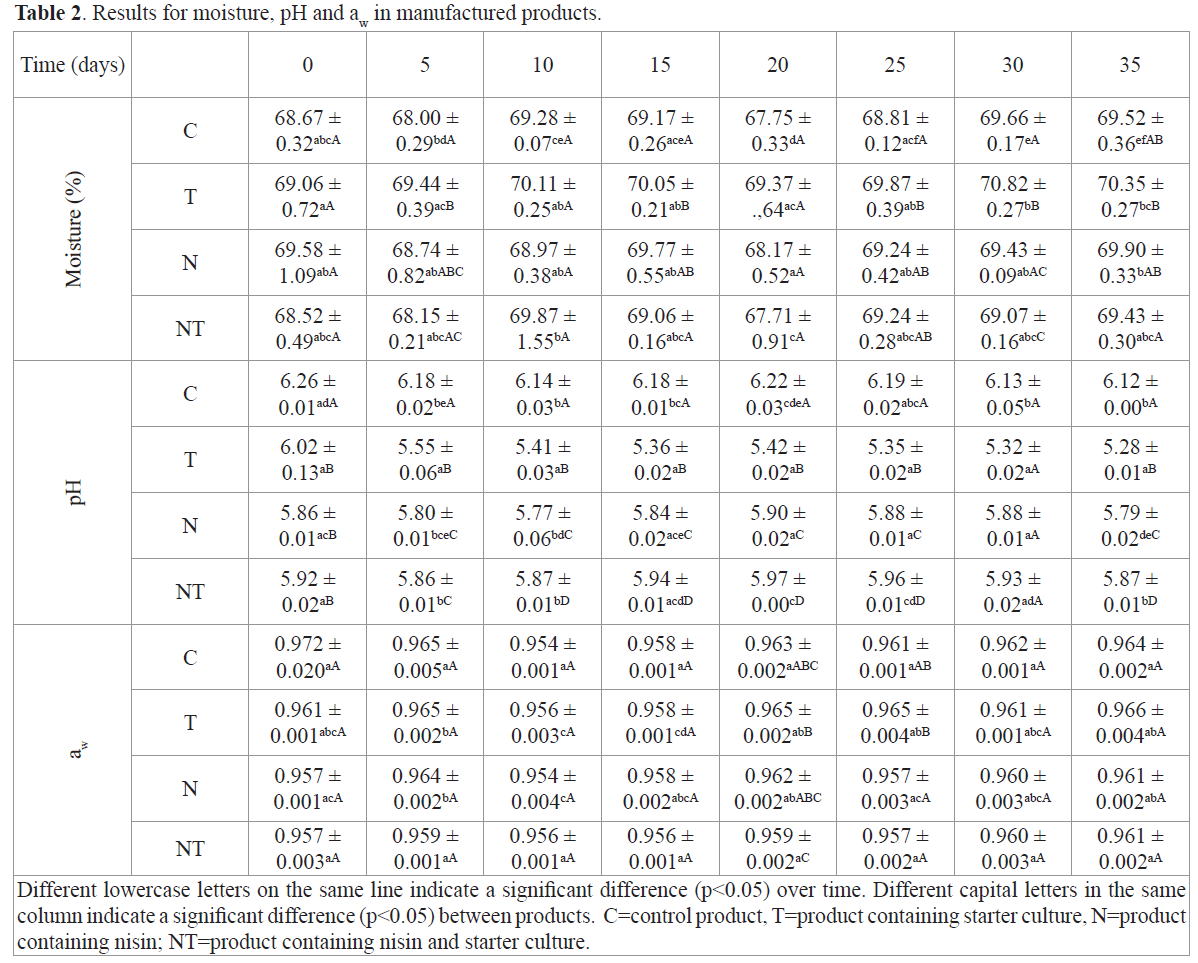
Table 2. Results for moisture, pH and aw in manufactured products.
The results of moisture showed some significant differences (p<0,05), especially over time. It’s important to note that the product that contains only starter culture (T), presented higher mean values in comparison to the others. Hu et al. (2008) observed the same effect studying goldfish sausages using various types of starter cultures containing combinations of Lactic Acid Bacteria and S. xylosus. These results can be justified by the fact that, during the catabolism of fatty acids promoted by microorganisms that are components of starter culture, a portion of water is released.
The product that received only starter culture experienced a sharp decline in pH. Riebroy et al. (2008) studying “song-fug”, a typical Thai fermented dish made from processed fish, observed that cultures containing LAB starters produced an effective decline in the pH of foods to which they were applied, due to the formation of organic acids that prevent the development of undesirable microorganisms in minimally processed foods. The authors also reported that the pH decline in the studied product was accelerated in the first 36 hours, reaching a minimum of 4.4. The same behavior was observed by Hu et al. (2008) in golden carp sausages containing starter cultures; however, a pH increase was observed in the product without starter culture (the control). Products containing nisin and nisin plus starter culture, presented significant differences (p<0,05) over time and with each other. However, pH didn’t decrease as it was observed in the product containing only starter culture. Zuckerman and Avraham (2002) treated salmon fillets with combinations of two types of bacteriocins, nisin and a commercial brand. The authors noted that bacteriocins alone were not sufficient to reduce pH. The dilution of nisin used in that study was made by acidifying the dilution water with phosphoric acid.The aw of Product C was not significantly different (p<0.05) over time, although differences were observed between the products at Times 20 and 25. Similar behavior was observed for Product T, but this behavior did not repeat over time. Products N and NT only presented significant differences (p<0.05) at Time 20. Hu et al. (2008) observed in their experiment that their products treated with starter culture had better aw than their control product; in the starter-culture products, aw was approximately 0.930 and dropped to a minimum of 0.820 after fermentation.
The results for the hardness parameter (Table 3) only differed over time for C and N and at Time 35. Riebroy et al. (2008) reported greater hardness in the song-fug they produced that received a special starter culture. They also stressed that hardness, such as occurs in fermented sausages, can be due to the denaturation and gelation of muscle proteins. This denaturation is due to reduced pH. Coelho et al. (2007) prepared surimi fishburgers with three different types of cassava starch in a proportion of 5%. They reported, based on several studies, that hardness is significant in restructured products with a meat/starch complex.
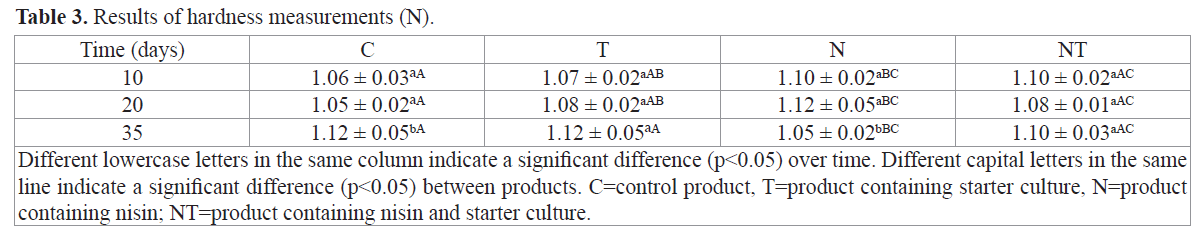
Table 3. Results of hardness measurements (N).
Microbiological analyses
The results of microbiological determinations in the raw material were within the limits specified by RDC 12, 2001 (Brazil, 2001), which established the maximum microbiological limits for each type of food. RDC 12 (Brazil, 2001) does not establish compliance parameters for total coliforms. However, this parameter is an indication of good storage and handling for any food product.Regarding the finished products, all results for the fecal coliform determination were < 3.0 MPN/g over the 35 days of the study. In relation to total coliforms, the results for Products T, N and NT were within the 95% confidence interval (p < 0.05) each time they were analyzed according Instruction Number 62 (Brazil, 2003). Product C, which contained no starter culture or nisin, had initial counts of MPN/g < 3 until it reached total coliforms MPN/g of > 1100 at Time 20, leading to its exclusion from the sensory sessions after that point. At other times, that value seemed to be decreasing, probably due to the increasingly poor survival conditions for the targeted microorganisms. No Salmonella spp was present in 25 g of any of the products. The results for coagulase-positive staphylococci were <1.0 × 102 CFU/g. These results are in accordance with RDC 12 (Brazil, 2001). Table 4 shows the results for the mesophilic bacteria determinations (CFU/g) for Products C, N, the psychrophilic bacteria counts (CFU/g) for Products C, N, NT and yeast and mold (CFU/g) for Products C, N, NT and T. RDC 12 (Brazil, 2001) doesn’t determine acceptance values for mesophilic bacteria and psychrophilic bacteria.
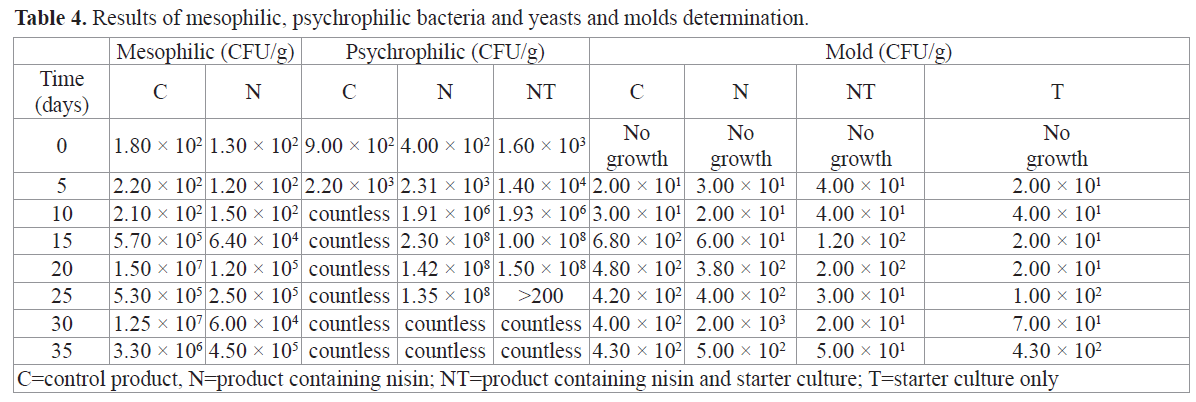
Table 4. Results of mesophilic, psychrophilic bacteria and yeasts and molds determination.
Product N, which contained nisin only, grew less mesophilic bacteria than the control, which had no barrier beyond the vacuum. A high count at 20 days (1.50 × 107 CFU/g), associated with the total coliform results, indicated that the control product had reached its limits for commercial life and could not be used. The nisin in Product N controlled the growth of mesophilic bacteria and was especially effective for controlling the growth of gram-positive bacteria. Hu et al. (2008) reported that carp sausages inoculated with starter culture showed an initial total count between log 3.7 and 4.7 CFU/g and reached the end of fermentation with stable or declining values ranging from log 2.3 CFU/g to log 9.6, depending on the product and the culture used. However, control products with no starter culture presented counts that increased from the beginning to the end of the analysis. The authors attributed these results to the pH decrease promoted by the presence of starter culture and bacteriocin.
The initial psychrophilic bacteria numbers for Product C could be counted, but they were no longer countable after Time 10. For Product N, psychrophilic bacteria could be counted until Time 30, but not after that point. Counts were possible for Product NT until Time 25. The products were handled manually during preparation, which, along with the presence of starter culture in Product NT, may have impacted these results. At Time 0, mold showed no CFU/g in any of the products. Mold growth was observed as early as Time 5. Product C showed the highest mold scores, followed by Product N, with a score of 2.00 × 103 at Time 30. The maximum mold value or Product T was 1.00 × 102 CFU/g at 25 and 35 days. Product NT grew more than 2.00 × 102 CFU/g in 20 days’ time.RDC 12 (Brazil, 2001) does not specify mold values for this type of product. Hu et al. (2008) observed growth behavior similar to the above results in their studies, in which the control product without starter culture showed an increase in mold growth over time. Products T and NT in our study remained virtually constant, even reaching regression values, as Hu et al. (2008) found in golden carp sausages. Thomas and Delves-Broughton (2005) emphasized that nisin is not effective in preventing mold and yeast growth. However, Dalié et al. (2010) reported that LAB cultures have the potential to control the mold development and, consequently, mycotoxin production. Among the species cited is L. curvatus, which is part of the starter culture that was used to prepare Products T and NT in the present study. In this case, the bacteria’s action would result from competition for nutrients, the production of organic acids and, in some cases, the production of other antagonist peptides. The results found in the products with added starter culture reinforce this evidence. Results of the counts of lactic acid bacteria (LAB) on MRS agar containing product of the starter culture (T and NT), are found in Table 5.
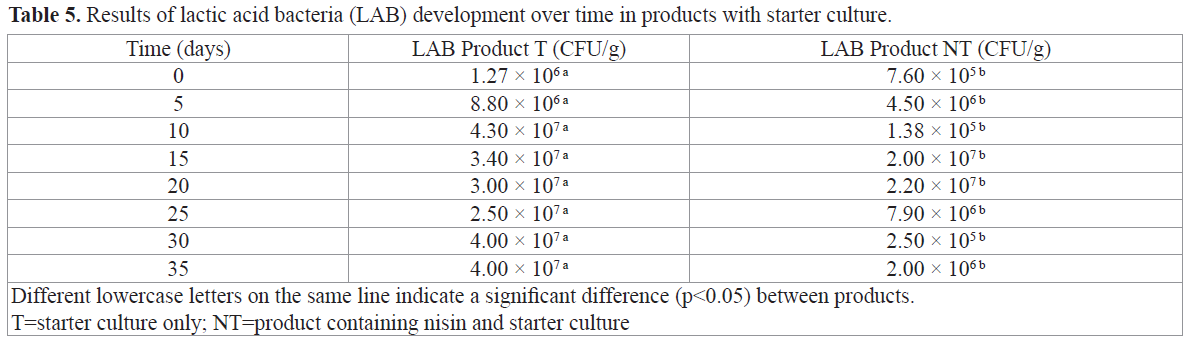
Table 5. Results of lactic acid bacteria (LAB) development over time in products with starter culture.
The results were analyzed using Student’s t test and indicated that the products were microbiologically different (p<0.05). Product NT had less LAB growth than Product T. This can be explained by the presence of nisin Product NT, which may have restricted the complete development of LAB because it is gram positive and nisin is very effective against these microorganisms (Chikindas et al., 2001; Rosa et al., 2002; Jay et al., 2005; Thomas and Delves-Broughton, 2005; Gálvez et al., 2007; Heng et al., 2007).
Conclusion
- Starter culture, nisin and/or a combination of both could be used in formulations of new products made of fish. Products containing only starter culture, had better pH reduction than those with nisin and starter culture plus nisin due to formation of organic acid. It was also evident the occurrence of lipolysis that product. The addition of nisin also showed potential use when associated with other barriers. The microbiological parameters set by Brazilian standards have been met. The delay in development of spoilage micro-organism has also been evidenced. In this way, it’s evident that starter cultures and bacteriocins of Lactic Acid Bacteria can be used, with other barriers, to increase the shelf life of products derived from fish.
6680
References
- nAraújo-Lima, C.A.R.M., Gomes, L.C. (2005). Tambaqui (Colossoma macropomum). In Espécies Nativas Para Piscicultura no Brasil, Federal University Santa Maria, Rio Grande do Sul, RGS, Brazil, pp. 34-56
- nAOAC (1997). Ofcial Methods of Analysis of the Association of Ofcial Analytical Chemist,, 16th ed., AOAC, Washington, DC
- nBrasil (1981). Métodos analíticos oficiais para controle de produtos de origem animal e seus ingredientes, Vol.2, No.11. Laboratório Nacional de Referência Animal. Ministério da Agricultura, Pecuária e do Abastecimento, Brasília, DF
- nBrasil (1997). Portaria n.185, de 13 de Maio de 1997, Regulamento técnico de identidade e qualidade de peixe fresco (inteiro e eviscerado), Ministério da Agricultura, Pecuária e do Abastecimento (MAPA), Brasília, DF
- nBrasil (2001). Ministério da Saúde, Agência Nacional de Vigilância Sanitária, Resolução (RDC) N° 12 de 02 de janeiro de 2001. Estabelece a regulamentação dos padrões microbiológicos para alimentos, Diário Oficial da União, 10 de Janeiro de 2001, 98pp. Brasília, DF
- nBrasil (2003). Instrução Normativa No. 62, de 26 de agosto de 2003, Oficializa os Métodos Oficiais para Análises Microbiológicas para Controle de Produtos de Origem Animal e Água, Anexo III, Secretaria de Defesa Agropecuária, Ministério da Agricultura, Pecuária e Abastecimento (MAPA), Brasília, DF
- nCalo-Mata, P., Arlindo, S., Boehme, K., de Miguel, T., Pascoal, A., Barros-Velazquez J. (2007). “Current applications and future trends of lactic acid bacteria and their bacteriocins for the biopreservation of aquatic food products”, Food Bioprocess Technology, Vol. 1, pp.43–63
- nCoelho, G.M., Weschendefelder, A.V., Meinert, E.M., Amboni, R.D.M.C., Beirão, L.H. (2007). “Effects of starch properties on textural characteristics of fish burgers: sensory and instrumental approaches”, Boletim do CEPPA, Vol.25, pp.37-50
- nCasaburi, A., Monaco, R., Cavella, S., Toldrá, F., Ercoline, D., Villani, F. (2008). “Proteolytic and lipolytic starter cultures and their effect on traditional fermented sausages ripening and sensory traits”, Food Microbiology, Vol. 25, pp.335-347
- nChagas, E.C, Gomes, L.C., Junior, H.M., Roubach R. (2007). “Produtividade de tambaqui criado em tanque-rede com diferentes taxas de alimentação”, Ciência Rural,Vol. 37, No.4, pp.109-1115
- nChikindas, M.L., Cleveland, J., Montville, T. J., Nes, I. F. (2001). “Bacteriocins: safe, natural antimicrobials for food preservation”, International Journal Food Microbiology, Vol.71, pp.1-20
- nDalié, D.K.D., Deschamps, A.M., Richard-Forget, F. (2010). “Lactic acid bacteria – potential for control of mould growth and mycotoxins: A Review”, Food Control, Vol. 21, pp.370-380
- nGálvez, A., Abriouel H., López, R.L., Omar, N.B. (2007). “Bacteriocin – based strategies for food biopreservation”, International Journal Food Microbiology, Vol. 120, pp.51-70
- nGonçalves, A.A., Nogueira, W.M., Lourenço, L.F.H. (2009). “Aproveitamento do descarte do processamento da piramutaba (Brachyplatystoma vaillantii) e do camarão-rosa (Farfantepenaeus subtilis) na produção de salsicha sabor camarão”, Boletim do Instituto de Pesca, Vol.35, pp.623– 635, available at: ftp://ftp.sp.gov.br/ftppesca/35_4_623-635. pdf. (accessed November 25, 2013)
- nHeng, N.C.K., Wescombe, P.A., Burton, J.A., Jack, R.W., Tagg, J.R. (2007). “The diversity of bacteriocins in gram-positive bacteria”, in Rileychavan, M.A. (Ed.) Bacteriocins, Ecology and Evolution, Springer, Nova York, pp. 34-138
- nHu,Y., Xia, W., Ge, C. (2008). “Characterization of fermented silver carp sausages inoculated with mixed starter culture”, LWT – Food Science and Technology, Vol.41, pp.730-738
- nJay, J.M., Loessner, M.J., Golden, D.A. (2005). Modern Food Microbiology, Springer, New York, NY, pp. 125-201,
- nKenneally, P.M., Leuschner, R.G., Arendt, E.K. (1998). “Evaluation of the lipolytic activity of starter cultures for meat fermentation purposes”, Journal Applied Microbiology, Vol.84, pp.839-846
- nKonings, W.N., Kok, J., Kuipers, O.P., Poolman, B. (2000). “Lactic acid bacteria: the bugs of new millennium, current opinion in microbiology”, Vol.3, pp. 276-282
- nLourenço, L.F.H, Galvão, G.C.S., Ribeiro, S.C.A., Ribeiro, C.F.A., Park, K.J. (2010). “Fat substitutes in processing of sausages using piramutaba waste”, Journal Food Science Technology, Vol. 47 No. 1. doi 10.1007/s13197-012-0645-8
- nMartines, E.C.P, Santarosa, P.R., Freitas, F.Z. (2003). “Caracterização preliminar de bacteriocinas produzidas por seis cepas de bactérias láticas isoladas de produtos cárneos embalados a vácuo”, Ciência e Tecnologia de Alimentos, Vol.23, pp.195-199. https://dx.doi.org/10.1590/S0101- 20612003000200016
- nNascimento, M.S., Moreno,I., Kuaie, A.Y. (2008). Bacteriocinas em Alimentos: uma Revisão, Brazilian Journal of Food Technology, Vol. 11, pp.120 – 127
- nOgawa, M.Y., Maia, E.L. (1999). Manual de pesca, Ciência e Tecnologia do Pescado. Varela, São Paulo, SP
- nOlesen, P., T., Stahnke, L.H. (2004). “The influence of environmental parameters on the catabolismo of branched–chain amino acids by Staphylococcus xylosus and Staphylococcus carnosus”, Food Microbiology, Vol. 21, 43-50
- nRiebroy, S., Benjakul, S., Visessanguan,W. (2008). “Properties and acceptability of som-fug, a thai fermented fish mince, inoculated with lactic acid bacteria starters”, LWT. Food Science and Technology, Vol.41, pp.569-580
- nRoos, N., Wahab, M.A., Chamnan, C., Thilsted, S.H. (2007). “The role of fish in food-based strategies to combat vitamin a and mineral deficiencies in developing countries”, Journal Nutrition, Vol.137, pp.1106–1109
- nRosa, C.M., Franco, B.D.G.M. (2002). Bacteriocinas de bactérias láticas. Conscientiae Saúde Uninove. Vol.1, pp.9-15, available at: https://pessoal.utfpr.edu.br/ leilamarques/arquivos/bacteriocinas.pdf (accessed January 16 2014)
- nSilva, N., Junqueira, V.C.A., Silveira, N.F.A., Taniwaki, M.H., Santos, R.F.S., Gomes, R.A.R. (2007). Manual de métodos de análises microbiológicas de alimentos, Varela, São Paulo, SP
- nTalon, R., Leroy, S. (2006). “Latest Developements in Meat Bacterial Starters”, in Nollet, M. L. M, Toldrá, F. (Ed), Advanced Technologies for Meat Processing, CRC Press, Boca Raton, pp.402-403
- nTakaya, S.L. ¾ükran Çakl¸la, S.C.D., Berna ±nç, K. (2003). “Quality changes of fish burger from rainbow trout during refrigerated”, Journal of Fisheries & Aquatic Sciences , Vol.20, pp.147 – 154
- nThomas, L.V., Delves-Broughton, J. (2005). “Nisin”, in Davidson, P. M., Sofos, J. N., Branen, A. L.(Ed.), Antimicrobials in Food, CRC Press, Boca Raton. pp.1-9
- nVanderzant, C., Splittstoesser, D.F. (1992). Compendium for microbiological examination of foods. American Public Health Association, Washington, DC
- nZuckerman,H., Avrahan, R.B. (2002). “Control of growth of Listeria monocytogenes in fresh salmon using MicrogardTM and Nisin”, LWT - Food Science and Technology, Vol.35, pp. 543–548.











