Key words
Corn gluten meal, Gilthead Sea Bream, Growth, Feed utilization
Introduction
Fish meal, the main protein source in aqua-culture diets, is the most important ingredient due to its high protein quality (Yigit et al., 2006). Considering that fish meal is the most expensive ingredient in fish diets, the increasing demand and instability of supply of this product forces feed manufacturers to reduce fish meal in the di-ets and use less expensive animal or plant protein sources as partial or total replacements for fish-meal. It has been reported that the global aqua-culture demand for fishmeal was 32% of the world supply level in 1999 (New and Wijkstöm, 2002), 37% in 2000 (Chamberlain, 2000) and it is estimated that this demand may reach nearly 70% by 2015 (New and Wijkstöm, 2002). If this trend continues to increase, soon the entire global fishmeal production might be used by the aqua-culture industry alone. This trend could also po-tentially reduce the profitability of fish culture as feed typically accounts for 35-60% of the pro-duction costs and moreover, the protein sources, where fishmeal is the most significant dietary in-gredient, account for about 50% of the total diet cost (Higgs, 1997). The sustainability of the growing aquaculture industry depends on the progressive reduction of wild fish catch as pro-tein source for aquaculture diets (Naylor, et al., 2000). In this perspective, aquaculture nutrition-ists have been attracted to investigate the possible reduction of dietary fishmeal and produce cost-effective, nutritionally balanced, and environ-ment friendly diets with alternative protein sources, supporting the sustainability of intensive aquaculture industry. However, due to the high protein requirements of carnivorous marine fishes, alternative protein sources such as animal or plant proteins are so far restricted to a few in-gredients with high-protein content, high digesti-bility and readily acceptable by the fish (Sargent and Tacon, 1999). One of the main problems in utilization of plant protein sources are the unbal-anced amino acid composition of the ingredient, and the anti-nutritional factors in most plant pro-teins (Krogdahl et al., 2003). For example corn gluten is known to be low in lysine or arginine, whereas soybean, the most commonly used plant protein source in aquaculture diets, is low in ly-sine and methionine, compared to fish meal sources and the requirement levels of fish (Hal-ver, 1991; NRC, 1993). Hence, the replacement of fish meal with a mixture of several plant pro-tein sources is a common approach in order to minimize the amino acid deficiencies in a for-mulated fish diet and meet the requirement levels of amino acid in fish species (De Francesco et al., 2007). Several studies are available on the re-placement of fish meal by CGM in diets for rain-bow trout (Gomes et al., 1995), yellowtail (Shimeno et al., 1993b), European sea bass (Ballestrazzi et al., 1994); Japanese flounder (Kikuchi, 1999); turbot (Regost et al., 1999; ti-lapia (Pereira and Oliva-Teles, 2003; Wu et al., 1995) and gilthead sea bream (Robaina et al., 1997; Ebiary, 2005).
It is well known that the utilization of plant protein sources is species or size specific in fish. The aim of this study was to evaluate the possi-bility of replacing a partial portion of fish meal in the diet for gilthead sea bream at early juvenile stage, right before or after their transfer to cage farms, using practical ingredients readily availa-ble to the aquaculture feed industry and to evalu-ate possible effects on growth performance, feed utilization, and economical inputs in early juve-nile fish.
Materials and Methods
Experimental diets
Four experimental diets, formulated from commercially available ingredients were pro-duced in the Fish Nutrition Laboratory on the Dardanos Campus of the Faculty of Fisheries at Canakkale Onsekiz Mart University. The diets were isonitrogenous and isocaloric with 52 % crude protein and 10 % lipid (19 kJ gross energy per g diet) (Table 1). In the control diet, brown fishmeal (anchovy meal) was the sole protein source. In the other three diets, fishmeal was re-placed by CGM at levels of 10%, 20%, or 30%. Amino acid profiles of the diets are given in Ta-ble 1; the proximate composition and amino acid profiles of the protein sources and gilthead sea bream are shown in Table 2; total n-3 HUFA contents of the diets, calculated according to Yigit et al. (2006) as follows are given in Table 3;
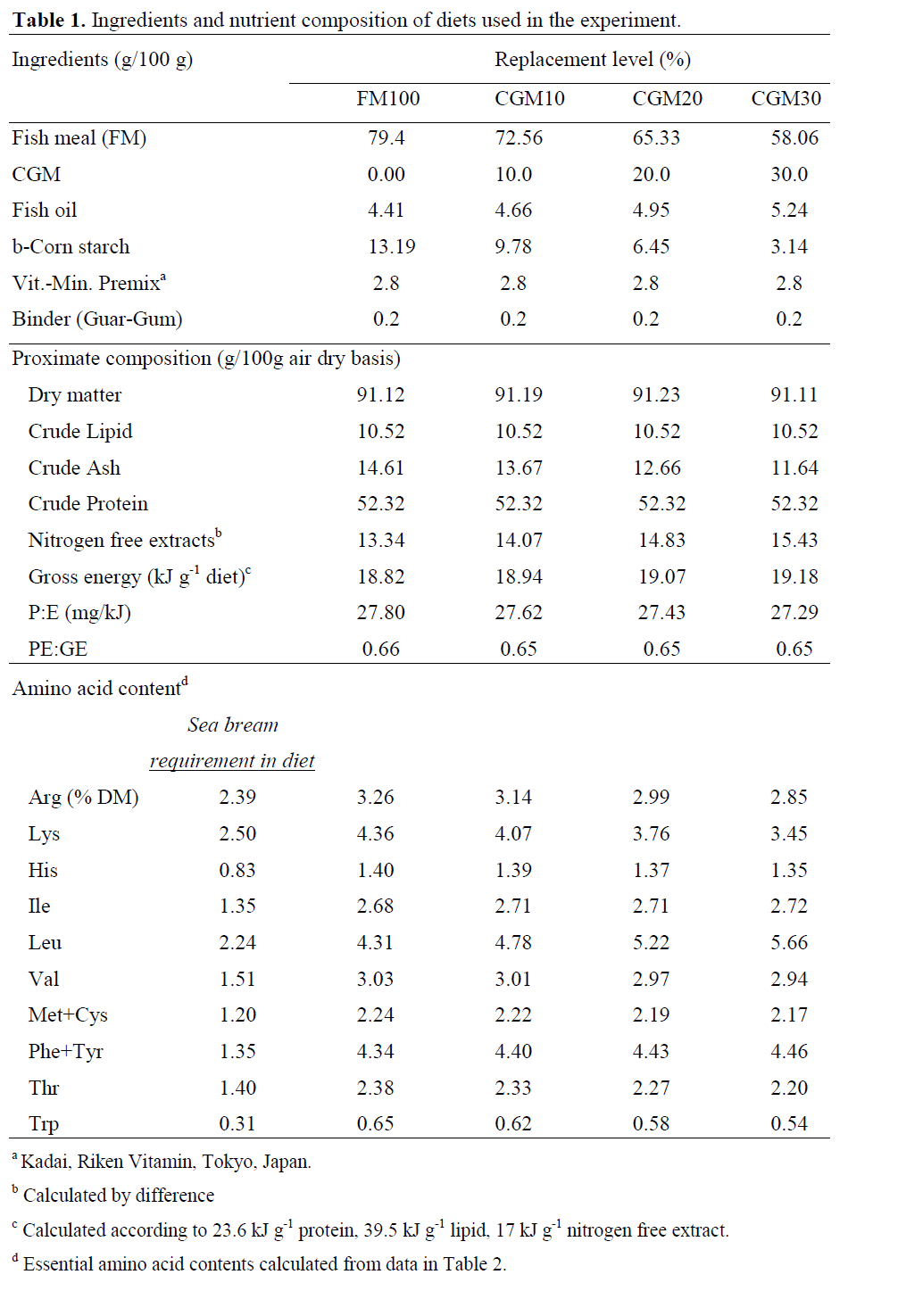
Table 1: The morphometric characters in the A.boyeri
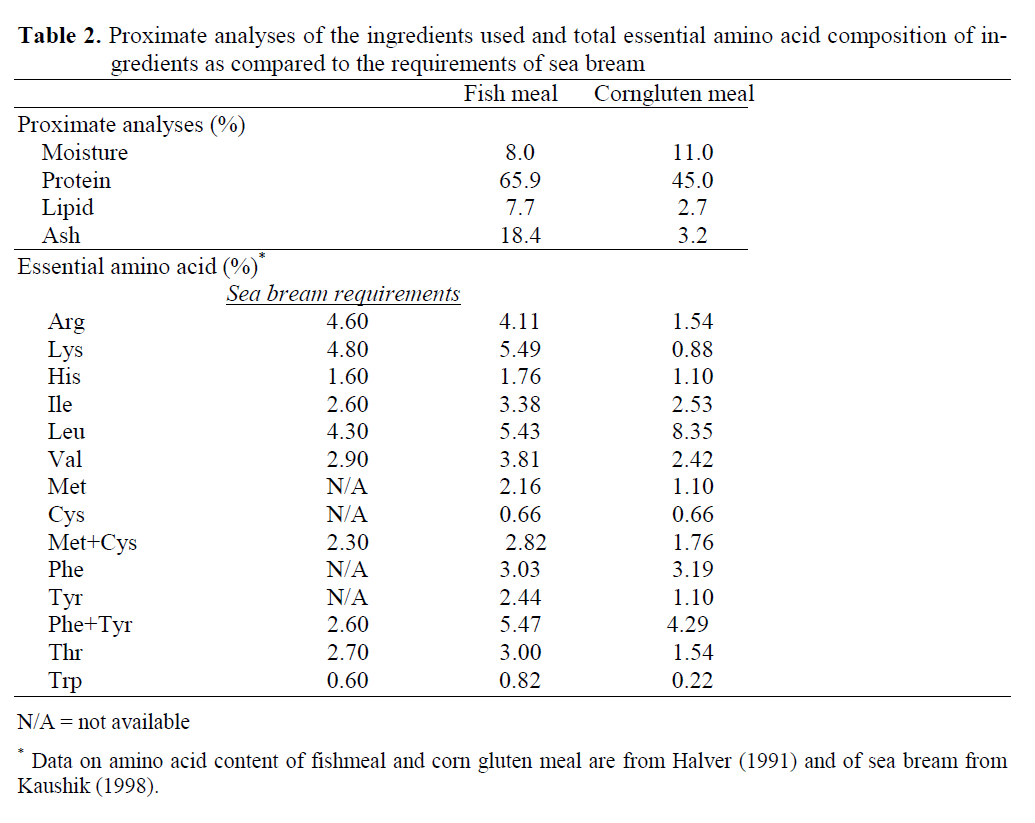
Table 2: Proximate analyses of the ingredients used and total essential amino acid composition of in-gredients as compared to the requirements of sea bream
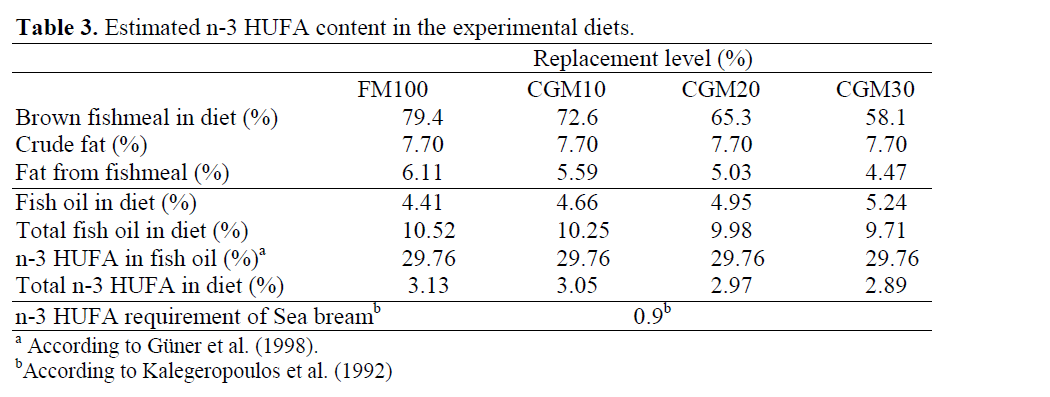
Table 3: Estimated n-3 HUFA content in the experimental diets.
Total n-3 HUFA contents= (total fish oil in g/kg diet) x (% n-3 HUFA in fish oil)
Dry ingredients and oil were mixed in a food mixer for 15 min. Tap water was blended into the mixture to attain a consistency suitable for pass-ing through a meat grinder with a 2-mm hole die. After pelleting, the diets were dried to a moisture content of 8-10% and stored at -20°C until use.
Growth trial and rearing conditions
Hatchery reared gilthead sea bream, Sparus aurata, were obtained from a commercial marine hatchery (IDA Gida Co.) in Canakkale, Turkey, and transported to the facilities of the Faculty of Fisheries of Canakkale Onsekiz Mart University in Canakkale, Turkey. After acclimation to the new environment, a total of 480 fish (1.53 ± 0.086 g initial mean weight) were randomly stocked into 12 identical 40-l rectangular poly-propylene blue colored tanks (40 fish per tank, three replicate tanks per treatment). The system was an indoor Recirculation Aquaculture System (RAS) run with sea water of 30.4 ± 1.3 g/l salin-ity. Continuous aeration was provided by air-stones. Fish were exposed to the natural light re-gime (40° 4'29.99" N, 26°21'35.58" E). The tanks were cleaned daily to remove uneaten feed and fecal material. Water quality was controlled peri-odically: pH ranged 7.5-8, total ammonia nitro-gen, determined by the Nessler method using a HANNA C200 portable spectrophotometer (HANNA Instruments, Co., Italy), ranged 0.24-0.29 mg/l, ambient water temperature ranged from 14.6 to 22.6 °C (18.2 ± 2.1°C) during the course of the study. Fish were hand fed twice daily at 09:00 and 17:00 for 45 days, from Au-gust to October 2010. Feeding activity was mon-itored carefully to ensure an even distribution of the feed to all fish in each tank. Fish were indi-vidually weighed at the start and end of the ex-periment, while group weighed on days 15, and 30. Fish were deprived of feed for one day prior to weighing.
Analytical methods
Experimental diets were chemically analyzed according to AOAC (1984) guidelines as follows: dry matter, by drying in an oven at 105°C for 24 h until a constant weight was obtained; protein (N x 6.25), by the Kjeldahl method after acid di-gestion; lipids, by ethyl ether extraction in a Soxhlet System; ash, by incineration in a muffle furnace at 550°C for 12 h; nitrogen free extracts, as the difference between total dry matter and (crude lipid + crude ash + crude protein).
Calculations
Following calculations on growth perfor-mance and feed utilization data were performed as described by Yigit et al. (2006);
Feed conversion rate (FCR) was calculated from the amount of feed consumed (dry matter) and the total biomass gained;
FCR = feed consumed / (weight gain + weight of dead fish)
Relative growth rate was calculated as the in-creased biomass in percent of the initial biomass;
RGR = [(final wet weight – initial wet weight) / initial wet weight] x 100
Specific growth rate (SGR) was calculated as percent increase of body weight per day;
SGR = [(ln final wet weight - ln initial wet weight) / days] x 100
Protein efficiency ratio (PER) was calculated as weight gain for each unit weigth of protein consumed;
PER = wet weight gain / protein intake
Daily feed intake was calculated as air dry feed consumed per fish per day;
DFI = (air dry feed intake/number of fish) / days
Likewise the daily protein and energy intake values were calculated as;
DPI = (feed intake x crude protein in diet / 100) / days
DEI = (feed intake x energy in diet / 100) / days
Statistical analysis
Results were analyzed by analysis of variance (ANOVA) using the PASW Statistical Analysis Software Program for Windows, Version 18.0.0, 2009, for significant differences among treatment means. Tukey test was used to detect significant differences (p<0.05) in growth performance data, feed intake, feed conversion rate, protein effi-ciency rate, and bio-economical data.
Results and Discussion
At the end of the growth trial, survival was 100% in all experimental groups, showing that partial replacement of FM by CGM in diets did not affect survival rates of fish. No significant difference (p>0.05) was recorded for final body weight (FBM) and for relative growth rate (RGR) among experimental fish fed the FM100 and CGM10 diets, which had the best growth rates (Table 4). Sea bream juveniles fed the CGM20 diet demonstrated a slightly lower FBW and RGR and the values were not significantly differ-ent from fish fed on CGM10 diet. When FM was further replaced by CGM, growth performance of fish showed significant reduction (p<0.05) in growth performance. Specific growth rate (SGR) followed the same trend, being best for the FM100 treatment group and poorest for CGM30, with significant similarity (p<0.05) between the FM100 and CGM10, the CGM10 and CGM20 and the CGM20 and CGM30 groups. Best feed conversion rate (FCR) values were found for fish fed diets containing FM only, and fish fed the 10% CGM has a GCR not significantly different (p>0.05) from those fed the FM based diet. Val-ues obtained for the protein efficiency rate (PER) was clearly higher for fish fed the FM100 and the CGM10 diets and showed a significant decrease (p<0.05) as fish meal was gradually replaced by CGM. No statistical differences (p<0.05) were found in daily feed intake, daily protein or energy intake per fish among experimental treatments (Table 4).
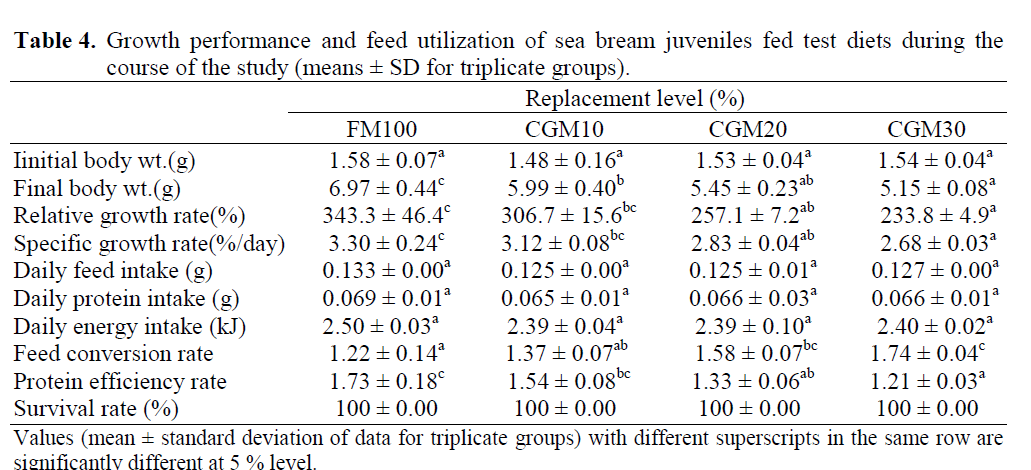
Table 4: Growth performance and feed utilization of sea bream juveniles fed test diets during the course of the study (means ± SD for triplicate groups).
Bio-economic analysis carried out for the ex-perimental diets showed that the Profit of the di-ets with no CGM inclusion (control) and the 10% CGM inclusion were significantly better than the other diets. Profit value of the 10% diet was not statistically different than the 20% CGM diet (p<0.05), but significantly different than the 30% CGM diet. The same relation was seen among experimental treatment groups when the feed cost values were calculated as percent of profit (Table 5). Hence, the profit from the treatment groups fed on 100% fish meal and the 10% CGM inclu-sion diets were significantly better than the other groups.
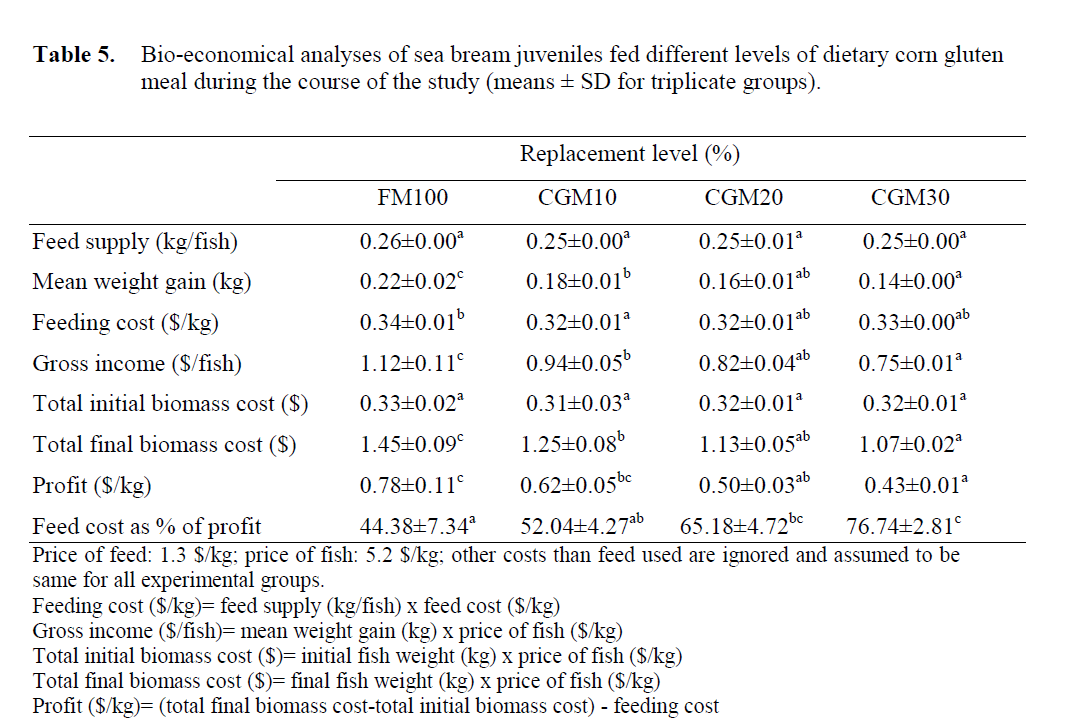
Table 5: Bio-economical analyses of sea bream juveniles fed different levels of dietary corn gluten meal during the course of the study (means ± SD for triplicate groups).
Growth performance obtained in the present study, were excellent for juvenile sea bream fed FM based and 10% CGM diets, however, a gra-dient decline was seen when dietary CGM in-creased up to 30% incorporation level, which is in agreement with other studies in terms of in-verse relationship between fish growth and die-tary level of CGM sources (Regost et al., 1999; Wu et al., 1995; Ebiary, 2005; Albrektsen et al., 2006). Daily feed intake, as well as daily protein or energy intake data were similar in all treatment groups, indicating that there were no palatability problems in the present study as fish in all groups readily accepted the experimental diets. Hence, the lower growth rates in the groups fed diets with higher levels of CGM over 10%, lead to in-creased FCRs, which can be attributed to the poorer utilization of the test diets with higher CGM inclusion over 10% under the conditions applied in this study.
Furthermore, the low utilization of higher CGM levels in the diets could be also due to the quality of raw material as for example the parti-cle size of the CGM used in this study was in some degree high. The poor growth performance of sea bream juveniles fed diets with higher lev-els of CGM were possibly because of the low bi-ological value of the CGM source used here, which is far too rich in leucine and marginal in lysine, arginine, and tryptophan when compared to FM in the present study. This also might be a possible reason that caused lower feed utilization. In contrast, some studies have shown considera-ble success in partial replacement of FM with CGM at levels of 12-26% in diets for trout (Alexis et al., 1985; Moyano et al., 1992), 20% for sea bass (Alliot et al., 1979), 40% for Japa-nese flounder (Kikuchi, 1999), 20% for turbot (Regost et al., 1999), and 60% for sea bream (Pe-reira and Oliva-Teles, 2003). It is important to note that crude protein content of CGM used in the present study was 45%, which is much lower than those used by Regost et al. (1999) (85.4%) and Pereira and Oliva-Teles (2003) (66.4%) crude protein, respectively, in diets for sea bream. It is noted that higher incorporation levels might be achieved by using ingredients with high-protein content (Pereira and Oliva-Teles, 2003). For example, several grain legumes have also been tested as alternative protein sources for fish, but their relatively low-protein content lim-ited the incorporation level to 20-30% of the di-etary protein (Carter and Hauler, 2000; Gouveia and Davies, 2000).
The discrepancy between the findings in the present study and those of previous ones in terms of the effect of CGM on growth performance and feed utilization might be attributed to several factors, namely; differences in diet composition, protein level and quality of the alternative plant protein source used, culture conditions such as water quality, temperature and salinity fluctua-tions, fish size or species, or a combination of these factors. In the present study, conducted for 45 days of feeding trial, there were no significant differences between experimental groups during the first 30 days of the experiment, however, visible changes in terms of growth appeared from day 30 onwards and significant differences have been recorded on day 45. Considering the growth trend of treatment groups in the present study, it can be deducted that more effective results could have been obtained when the experiment would have continued after 45 days of trial, which also could be a one of the reasons for the lower utili-zation of CGM in this study compared to those in the previous ones. Overall, our results in terms of growth performance and feed utilization data of sea bream fed increasing levels of CGM are comparable to those of previous studies on sea bream nutrition (Robaina et al., 1997; Fournier et al., 2002; Pereira and Oliva-Teles, 2003; Pereira and Oliva-Teles, 2002; De Francesco et al., 2007).
In general, CGM is known to have an appro-priate amino acid balance for marine fish species, however, amino acid contents of the test diets in the present study, particularly lysine and arginine were lower than those used in previous studies mentioned above. Furthermore, some of the pre-vious studies (Davies et al., 1997; Kikuchi, 1999; Regost et al., 1999; Cheng et al., 2003; Fournier et al., 2004; Albrektsen et al., 2006; De Fran-cesco et al., 2007) have supplemented the ex-perimental diets with amino acids (mainly lysine and arginine), which may have contributed to an increased replacement level. In this study, how-ever, diets were not fortified with amino acids, and also the crude protein content of CGM was lower than the previous studies, which also might have affected reduced growth performance of fish in the present study.
Even though the experimental diets in the pre-sent study were not fortified with essential amino acids, the required levels of amino acid for sea bream were provided by the experimental diets and met the requirements of sea bream reported by Kaushik (1998). However, there were still im-balances of AA contents among each test diet. Nevertheless, the imbalances in the amino acid composition between experimental groups were explicit. For example, the 20% and 30% CGM diets contained 14% and 20% less lysine, respec-tively than the control diet. Lysine is generally considered the first limiting amino acid in most fish species (Robaina et al. 1997), and both lysine and arginine are the two main limiting amino acids in CGM for aquaculture feeds (Amerio et al., 1998), which was reflected in the amino acid profile of the experimental diets with increasing levels of CGM. In the present study, besides ly-sine, arginine content in the 20% and 30% CGM diets were also less (8% and 13%, respectively) than the control diet. Therefore, the 22 to 26% lower mean final weights in the 20% or 30% CGM groups could be attributed to the lower ly-sine or arginine contents of the test diets com-pared to the control group.
All the experimental diets met the essential fatty acid requirements of sea bream. However, due to the lower amount of anchovy oil and lipids provided by the lower amount of fishmeal, the experimental diets contained less n-3 HUFA than the control. The 5% to 8% differences of n-3 HUFA content in the 20% and 30% CGM diets, respectively, compared to the reference diet could explain the differences in growth performance. Furthermore, it is well known that the quality of plant protein sources can be improved by thermal treatment and solvent-extraction (Burel et al., 2000). Based on the estimated n-3 HUFA and amino acid contents in the test diets, the poorer growth of fish fed over 10% replacement diets could be attributed to the variation of HUFA or amino acids among treatment groups, as well as to processing conditions, quality of the ingredi-ents, poor digestibility or palatability, or to the combination of these factors.
Economical analyses also confirmed the growth experimental results in terms of best profit obtained with the 10% CGM inclusion diet and the worst with the highest dietary CGM of 30%. The profit from fish fed on 100% fish meal and the 10% CGM inclusion diets demonstrated better results in terms of cost-effective sea bream culture with CGM replacing fish meal in the diet.
Conclusion
As a conclusion, based on the findings in the present study, CGM can be incorporated up to 10% in diets for gilthead sea bream juveniles, with no adverse effects on growth performance, feed utilization or economical inputs, even with both low protein level and low quality raw mate-rial, and without any amino acid supplementa-tion. It is suggested to use high quality raw mate-rial of CGM with higher protein content when replacing fish meal in aquafeeds. Furthermore, it also seems to be worth investigating the potential of dietary CGM in combination with other plant protein sources as a partial replacement for fish meal in diets for European sea bream in order to avoid any dietary imbalance of the amino acid composition and produce cost-effective and envi-ronment friendly feeds for a sustainable future of the aquaculture industry.
Acknowledgements
The financial support by the Scientific Re-search Commission (BAP-Bilimsel Arastirma Komisyonu, Project No. BAP-2009/43) of Canakkale Onsekiz Mart University, Turkey, is greatfully acknowledged. We would like to thank IDA Gida Co. in Canakkale, Turkey for support-ing the experimental animals for this study.
679
References
- Albrektsen, S., Mundheim, H., Aksnes, A., (2006). Growth, feed efficiency, digestibility and nutrient distribution in Atlantic cod (Gadusmorhua) fed two different fish meal qualities at three dietary levels of vegetable protein sources. Aquaculture, 261: 626-640. doi: 10.1016/j.aquaculture.2006.08.031
- nAlexis, M.N, Papaparaskeva-Papoutsoglou, E., Theochari, V., (1985). Formulation of prac-tical diets for rainbow trout (Salmogaird-neri) made by partial or complete substitu-tion of fish meal by poultry by-products and certain plant by-products. Aquaculture, 50: 61-73. doi: 10.1016/0044-8486(85)90153-X
- nAlliot, E., Pastoreaud, A., Pelaez, J., Métailler, R., (1979). Utilization des farineavégétaleset des levurescultivéessuralcanes pour l’alimentation du bar (Dicentrurchuslabrax), Vol. II. Proc. World Symp. on Fin-fish Nutrition and Fishfeed Technology, Hamburg, 20-23 June, 1978, pp. 229-238
- nAmerio, M., Vignali, C., Castelli, L., Fiorentini, L., Tibaldi, E., (1998). Vegetable protein sources, protein evaluation indexes and ‘ideal protein’of sea bream (Sparusaurata). Rivistataliana di Acquacoltura, 33: 135-145
- nAOAC, (1984) Official Methods for Analysis, 14th edn. Association of Official Analytical Chemists, Washington, USA
- nBallestrazzi, R., Lanari, D., Dagaro, E., Mion, A., (1994). The effect of dietary protein level and source on growth, body composition, total ammonia and reactive phosphate ex-cretion of growing sea bass (Dicentrarchuslabrax). Aquaculture, 127: 197-206. doi: 10.1016/0044-8486(94)90426-X
- nBurel, C., Boujard, T., Kaushik, S.J., Boeuf, G., Van Der Geyten, S., Mol, K.A., Kuhn, E.R., Quinsac, A., Krouti, M., Ribaillier, D., (2000). Potential of plant-protein sources as fish meal substitutes in diets for turbot (Psetta maxima): growth, nutrient utilisation and thyroid status. Aquaculture, 188: 363-382. doi: 10.1016/S0044-8486(00)00342-2
- nCarter, C.G., Hauler, R.C., (2000). Fish meal re-placement by plant meals in extruded feeds for Atlantic salmon, SalmosalarL. Aqua-culture, 185: 299-311. doi: 10.1016/S0044-8486(99)00353-1
- nChamberlain, G.W., (2000). Aquaculture projec-tions for use of fishmeal and oil. Oral presentation at the Annual Meeting of FOMA, Lima, Peru, 30 October-3 Novem-ber 2000
- nCheng, Z.J., Hardy, R.W., Usry, J.L., (2003). Ef-fects of lysine supplementation in plant protein-based diets on the performance of rainbow trout (Oncorhynchusmykiss) and apparent digestibility coefficients of nutri-ents. Aquaculture, 215: 255-265. doi: 10.1016/S0044-8486(02)00166-7
- nDavies, S.J., Morris, P.C., Baker, R.T.M., (1997). Partial substitution of fishmeal and full fat soyabean meal with maize gluten and influ-ence of lysine supplementation in diets for the rainbow trout OncorhynchusmykissWalbaum. Aquaculture Research, 28: 317-328. doi:10.1111/j.1365-2109.1997.tb01048.x
- nDe Francesco, M., Parisi, G., Perez-Sanchez, J., Gomez-Requeni, P., Medale, F., Kaushik, S.J., Mecatti, M., Poli, B.M., (2007). Effect of high-level fish meal replacement by plant proteins in gilthead sea bream (Sparus au-rata) on growth and body/fillet quality traits. Aquaculture Nutrition, 13: 361-372. doi: 10.1111/j.1365-2095.2007.00485.x
- nEbiary, EH., (2005). Use of soybean meal and/ or corn gluten meal as Partial substitutes for fish meal in Nile tilapia (Oreochromisni-loticus) fingerling diets. Egyptian Journal of Aquatic Research, 31: 432-442
- nFournier, V., Gouillou-Coustans, M.F., Métailler, R., Vachot, C., Guedes, M.J., Tulli, F., Oliva-Teles, A., Tibaldi, E., Kaushik, S.J., (2002). Protein and arginine requirements for maintenance and nitrogen gain in four teleosts. British Journal of Nutrition, 87: 459-469. doi: 10.1079/BJN2002564
- nFournier, V., Huelvan, C., Desbruyeres, E., (2004). Incorporation of a mixture of plant feedstuffs as substitute for fish meal in diets of juvenile turbot (Psetta maxima). Aqua-culture, 236: 451-465. doi: 10.1016/j.aquaculture.2004.01.035
- nGomes, E.F., Rema, P., Kaushik, S.J., (1995). Replacement of fish meal by plant proteins in the diet of rainbow trout (Oncorhynchusmykiss): digestibility and growth perfor-mance. Aquaculture, 130: 177-186. doi: 10.1016/0044-8486(94)00211-6
- nGouveia, A., Davies, S.J., (2000). Inclusion of an extruded dehulled pea seed meal in diets for juvenile European sea bass (Dicentrarchuslabrax). Aquaculture, 182: 183-193. doi: 10.1016/S0044-8486(99)00246-X
- nGüner, S., Dincer, B., Alemdag, N., Colak, A., Tufekci, M., (1998). Proximate composition and selected mineral content of commer-cially important fish species from the Black Sea. Journal of the Science of Food and Ag-riculture, 78: 337-342. doi:10.1002/(SICI)1097-0010(199811)78:3<337::AID-JSFA122>3.0.CO;2-A
- nHalver, J.E., (1991). Fish Nutrition. 2nd ed. Aca-demic Press, California, USA
- nHiggs, D.A., (1997). Nutritional strategies for cost effective salmon production. In: Pro-ceedings of the First Korea-Canada Joint Symposium in Aquatic Biosciences, October 16, 1997, pp. 67-91. Institute of Fisheries Sciences, Pukyong National University, Pu-san, South Korea
- nKalegeropoulos, N., Alexis, M., Henderson, R.J., (1992). Effect of dietary soybean and cod-liver oil levels on growth and body compo-sition of gilthead bream (Sparusaurata). Aquaculture, 104: 293-308. doi: 10.1016/0044-8486(92)90211-3
- nKaushik, S.J., (1998). Whole body amino acid composition of European seabass (Dicen-trarchuslabrax), gilthead seabream (Sparusaurata) and turbot (Psetta maxima) with an estimation of their IAA requirement profiles. Aquatic Living Resources, 11: 355-358. doi: 10.1016/S0990-7440(98)80007-7
- nKikuchi, K., (1999). Partial Replacement of Fish Meal with Corn Gluten Meal in Diets for Japanese Flounder Paralichthysolivaceus. Journal of the World Aquaculture Society, 30: 357-363. doi:10.1111/j.1749-7345.1999.tb00686.x
- nKrogdahl, A., Bakke-McKellep, A.M., Baever-fjord, G., (2003). Effects of graded levels of standard soybean meal on intestinal struc-ture, mucosal enzyme activities, and pancre-aticresponse in Atlantic salmon (SalmosalarL.). Aquaculture Nutrition, 9: 361-371. doi: 10.1046/j.1365-2095.2003.00264.x
- nMoyano, F.J., Cardenete, G., De la Higuera, M., (1992). Nutritive value of diets containing high percentage of vegetable proteins for trout. Oncorhynchusmykiss. Aquatic Living Resources, 5: 23-29. doi: 10.1051/alr:1992004
- nNaylor, R.L., Goldburg, R.J., Primavera, J.H., Kautsky, N., Beveridge, M.C.M., Clay, J., Folke, C., Lubchenco, J., Mooney, H., Tro-ell, M., (2000). Effect of aquaculture on world fish supplies. Nature, 405: 1017-1024. doi: 10.1038/35016500
- nNew, M.B., Wijkstöm, U.N., (2002). Use of fishmeal and fish oil in aquafeeds: further thoughts on the fishmeal trap. FAO Fisher-ies Circular, No. 975 FIPP/C975. Food and Agriculture Organization of the United Na-tions, Rome
- nNRC, (1993). Nutrient Requirement of Fish. Na-tional Academy Press, Washington, DC, USA
- nPereira, T.G., Oliva-Teles, A., (2002). Prelimi-nary evaluation of pea seed meal in diets for gilthead sea bream (Sparusaurata) juve-niles. Aquaculture Research, 33: 1183-1189. doi: 10.1046/j.1365-2109.2002.00782.x
- nPereira, T.G., Oliva-Teles, A., (2003). Evaluation of corn gluten meal as a protein source in diets for gilthead sea bream (SparusaurataL.) juveniles. Aquaculture Research, 34: 1111-1117. doi: 10.1046/j.1365-2109.2003.00909.x
- nRegost, C., Arzel, J., Kaushik, S.J., (1999). Par-tial or total replacement of fish meal by corn gluten meal in diet for turbot Psetta maxima. Aquaculture, 180: 99-117. doi: 10.1016/S0044-8486(99)00026-5
- nRobaina, L., Moyano, F.J., Izquierdo, M.S., So-corro, J., Vergara, J.M., Montero, D., (1997). Corn gluten and meat and bone meals as protein sources in diets for gilthead seabream (Sparusaurata): nutritional and histological implications. Aquaculture, 157: 347-359. doi: 10.1016/S0044-8486(97)00174-9
- nSargent, J.R., Tacon, A.G.J., (1999). Develop-ment of farmed fish: a nutritionally neces-sary alternative to meat. Proceedings of the Nutrition Society, 58: 377-383. doi: 10.1017/S0029665199001366
- nShimeno, S., Mima, T., Imanaga, T., Tomaru, K., (1993b). Inclusion of combination of defat-ted soybean meal, meat meal, and corn glu-ten meal to yellowtail diets. Nippon Suisan Gakkaishi, 59: 1889-1895. doi: 10.2331/suisan.59.1889
- nWu, Y.V., Rosati, R.R., Sessa, D.J., Brown, P.B., (1995). Evaluation of corn gluten meal as a protein source in tilapia diets. Journal of Ag-riculture Food Chemistry, 43: 1585-1588. doi: 10.1021/jf00054a032
- nYigit, M., Erdem, M., Koshio, S., Ergün, S., Tür-ker, A., Karaali, B., (2006). Substituting fish meal with poultry by-product meal in diets for Black Sea turbot Psettamaeotica, Aqua-culture Nutrition, 12: 340-347. doi: 10.1111/j.1365-2095.2006.00409.x











